Question: Please explain why it is the better nucleophile in each case only for questions d.) - h.) - Which is a better nucleophile: a) Bror
 Please explain why it is the better nucleophile in each case only for questions d.) - h.)
Please explain why it is the better nucleophile in each case only for questions d.) - h.)
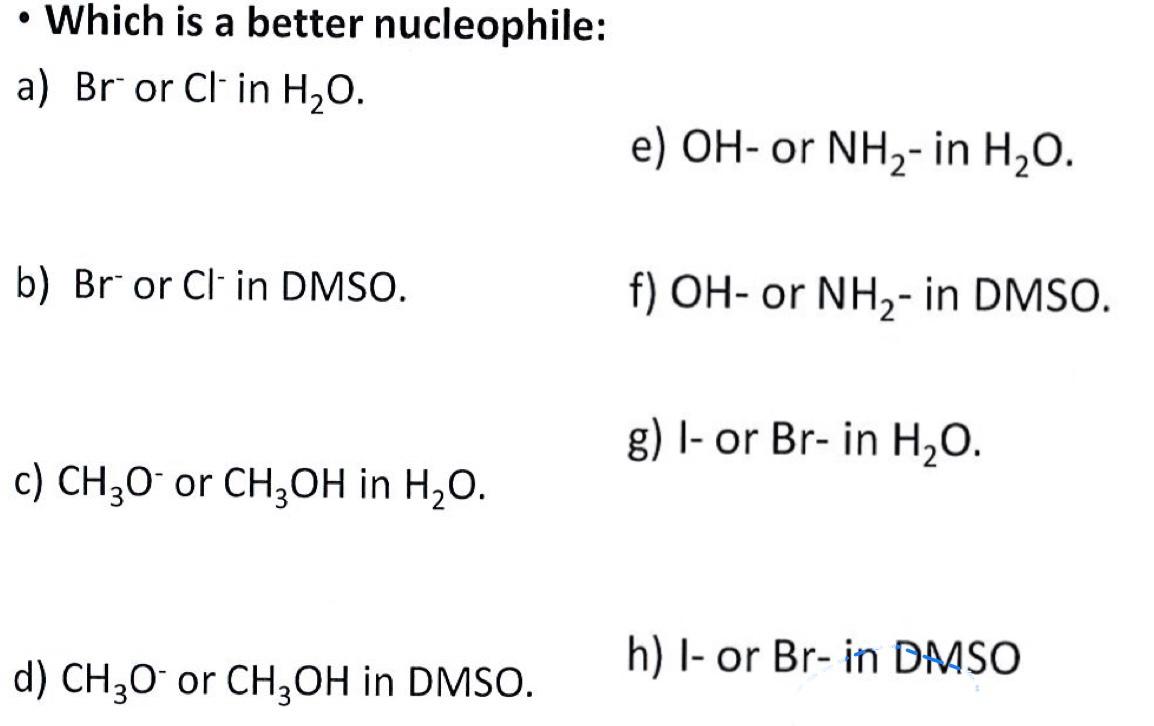
- Which is a better nucleophile: a) Bror Clin H2O. e) OH - or NH2 - in H2O. b) Bror Clin DMSO. f) OH - or NH2 - in DMSO. c) CH3Oor CH3OH in H2O. g) I- or Br - in H2O. d) CH3Oor CH3OH in DMSO. h) I- or Br - in DASO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


