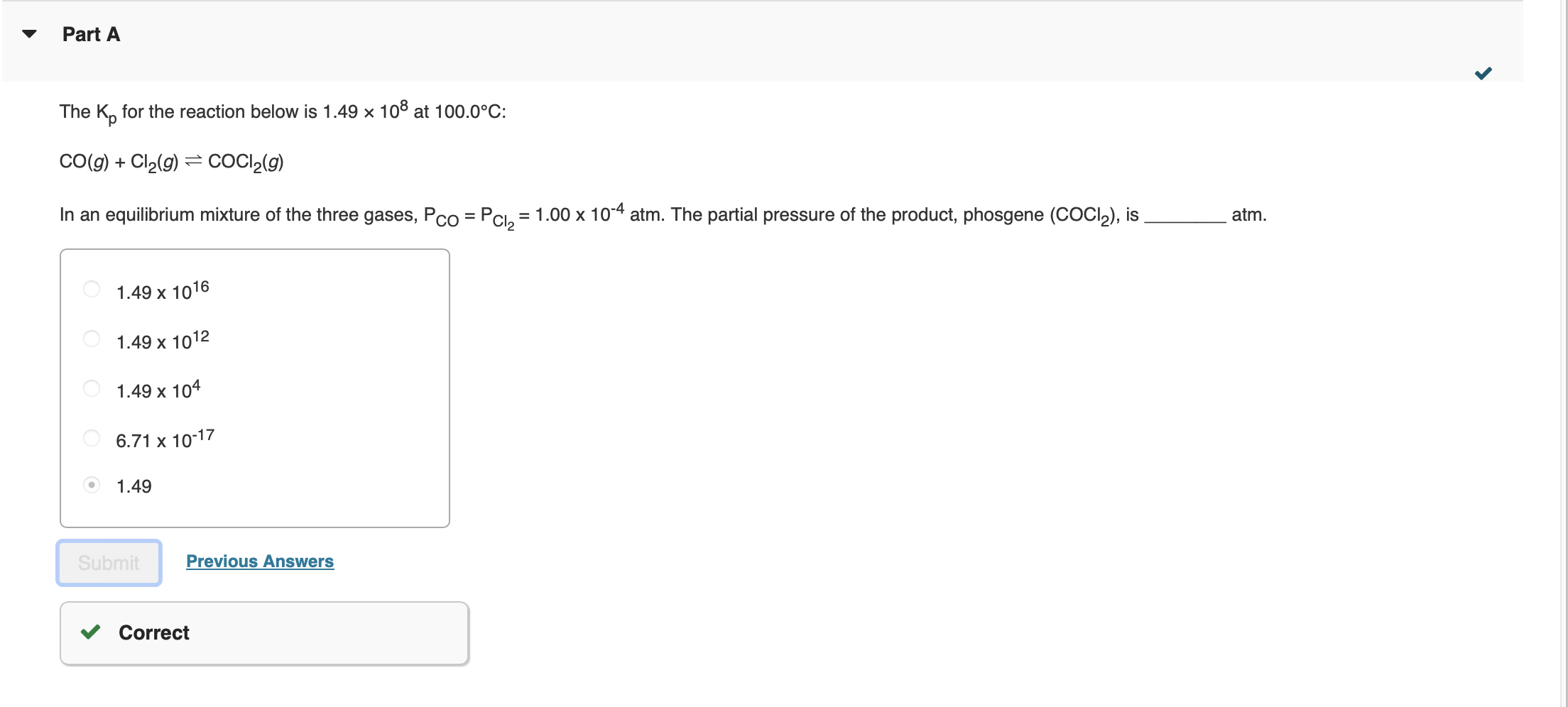
Question: Please explain why the answer is 1.49 not a 1.49 * 10^16. The Kp for the reaction below is 1.49108 at 100.0C : CO(g)+Cl2(g)COCl2(g) In
 Please explain why the answer is 1.49 not a 1.49 * 10^16.
Please explain why the answer is 1.49 not a 1.49 * 10^16.
The Kp for the reaction below is 1.49108 at 100.0C : CO(g)+Cl2(g)COCl2(g) In an equilibrium mixture of the three gases, PCO=PCl2=1.00104atm. The partial pressure of the product, phosgene (COCl), is 1.4910161.4910121.491046.711017 1.49
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


