Question: please explain with clear handwriting I5L Problem One (35 Points) A 15-liter rigid vessel, filled to one-third its volume with liquid nitrogen at its normal
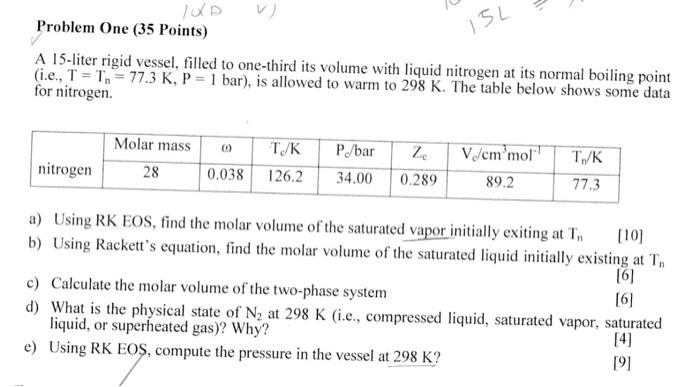
I5L Problem One (35 Points) A 15-liter rigid vessel, filled to one-third its volume with liquid nitrogen at its normal boiling point (i.e., T = T, = 77.3 K, P = 1 bar), is allowed to warm to 298 K. The table below shows some data for nitrogen. Molar mass 28 Z nitrogen 00 0.038 TIK 126.2 P/bar 34.00 V/cm'mol' 89.2 T./K 77.3 0.289 a) Using RK EOS, find the molar volume of the saturated vapor initially exiting at T, [10] b) Using Rackett's equation, find the molar volume of the saturated liquid initially existing at T [6] c) Calculate the molar volume of the two-phase system [6] d) What is the physical state of N, at 298 K (i.e., compressed liquid, saturated vapor, saturated liquid, or superheated gas)? Why? [4] e) Using RK EOS, compute the pressure in the vessel at 298 K? [9]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


