Question: please solve on paper and show steps Une (35 Points) A 15-liter rigid vessel, filled to one-third its volume with liquid nitrogen at its normal
 please solve on paper and show steps
please solve on paper and show steps
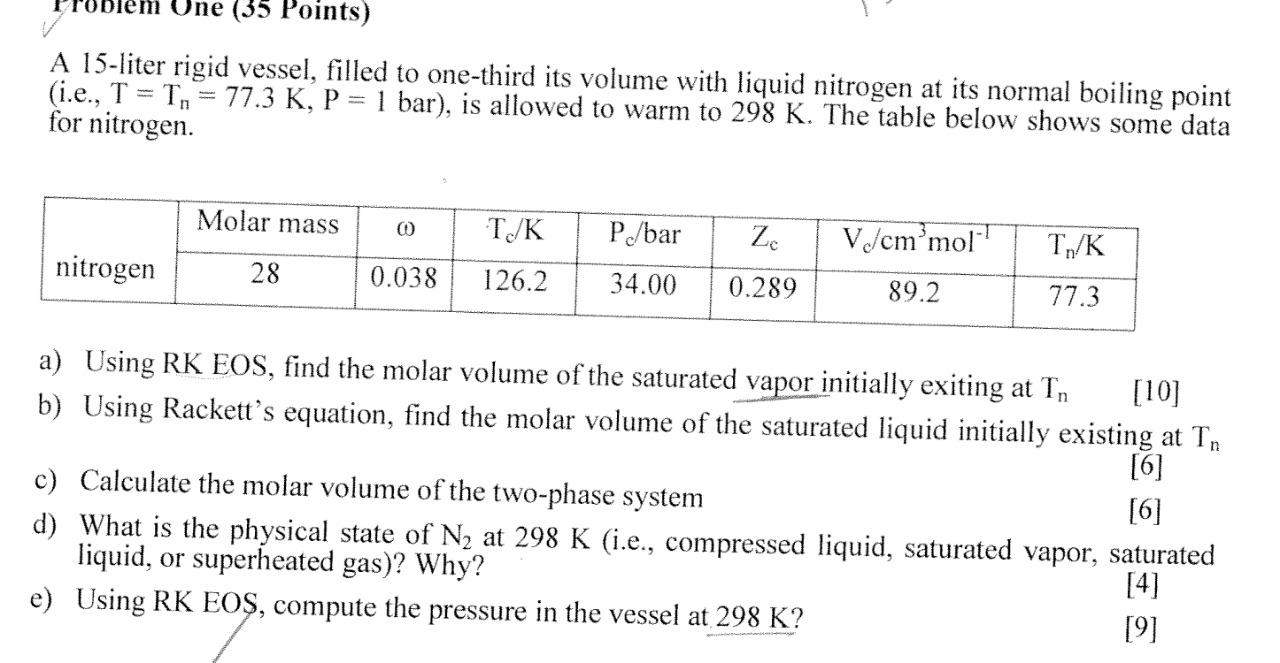
Une (35 Points) A 15-liter rigid vessel, filled to one-third its volume with liquid nitrogen at its normal boiling point (i.e., T = T, = 77.3 K, P = 1 bar), is allowed to warm to 298 K. The table below shows some data for nitrogen. Molar mass 0 T/K P/bar Zc V/cm'mol nitrogen 28 0.038 126.2 34.00 0.289 89.2 77.3 a) Using RK EOS, find the molar volume of the saturated vapor initially exiting at Tn [10] b) Using Rackett's equation, find the molar volume of the saturated liquid initially existing at Tn [6] c) Calculate the molar volume of the two-phase system [6] d) What is the physical state of N2 at 298 K (i.e., compressed liquid, saturated vapor, saturated liquid, or superheated gas)? Why? [4] e) Using RK EOS, compute the pressure in the vessel at 298 K? [9]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


