Question: Please explain your solutions and show all work even if its simple integration. Thank you! I promise I will like if it is correct. Problem
Please explain your solutions and show all work even if its simple integration. Thank you! I promise I will like if it is correct.
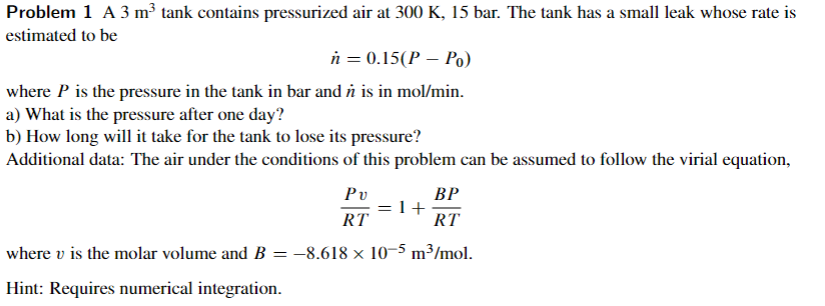
Problem 1 A 3 m tank contains pressurized air at 300 K, 15 bar. The tank has a small leak whose rate is estimated to be n = 0.15(P P.) where P is the pressure in the tank in bar and n is in mol/min. a) What is the pressure after one day? b) How long will it take for the tank to lose its pressure? Additional data: The air under the conditions of this problem can be assumed to follow the virial equation, BP =l+ RT RT where v is the molar volume and B = -8.618 x 10-5 m3/mol. Hint: Requires numerical integration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


