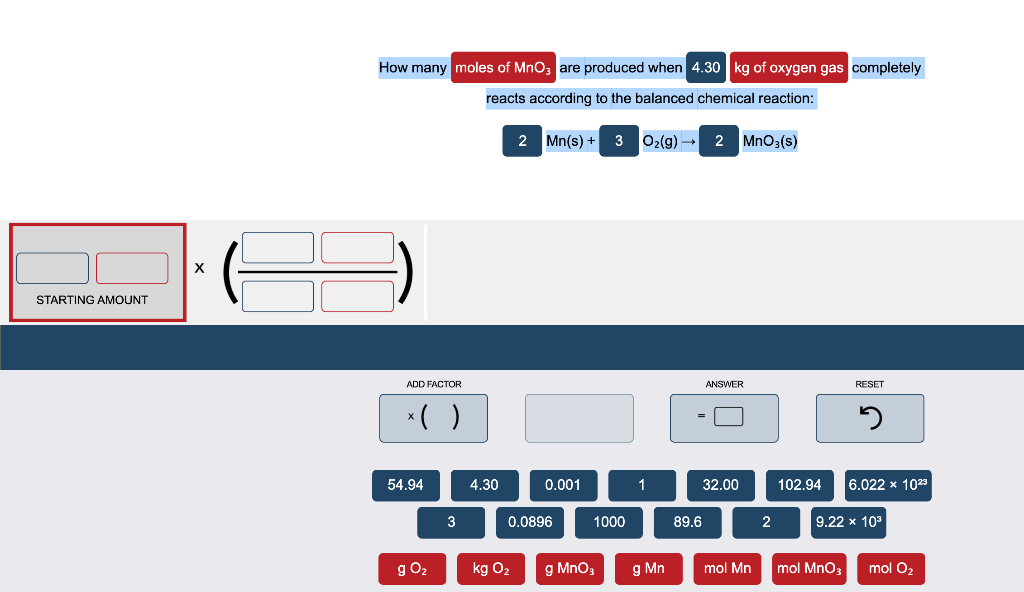
Question: Please fill in the boxes, I always have a hard time with the conversion table. Thank you. How many moles of Mnoz are produced when
 Please fill in the boxes, I always have a hard time with the conversion table. Thank you.
Please fill in the boxes, I always have a hard time with the conversion table. Thank you.
How many moles of Mnoz are produced when 4.30 kg of oxygen gas completely reacts according to the balanced chemical reaction: 2 Mn(s) + 3 O2(g) 2 MnO3(s) STARTING AMOUNT ADD FACTOR ANSWER RESET x( ) 2 54.94 4.30 0.001 1 32.00 102.94 6.022 x 1023 3 0.0896 1000 89.6 2 9.22 x 103 g 02 kg 02 g Mno g Mn mol Mn mol Mnog mol O2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


