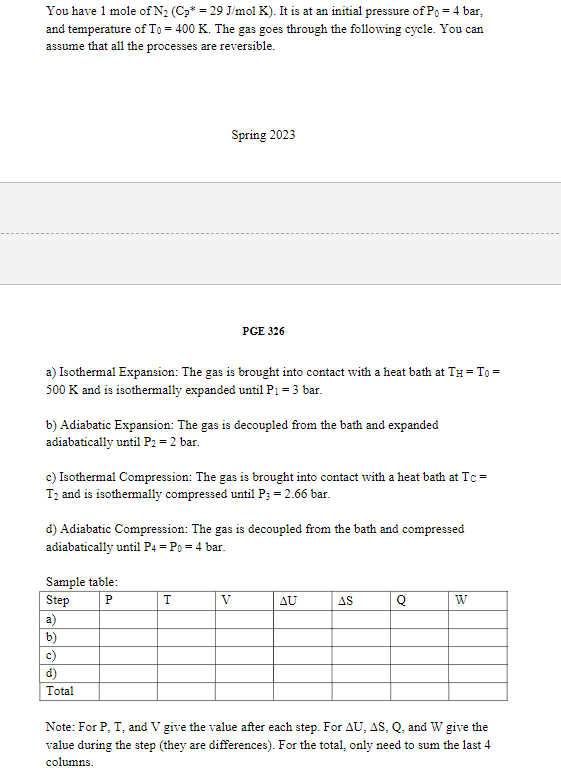
Question: Please fill in the SAMPLE TABLE! Please! You have 1 mole of N2(Cp=29J/molK). It is at an initial pressure of P0=4 bar, and temperature of
Please fill in the SAMPLE TABLE! Please!
You have 1 mole of N2(Cp=29J/molK). It is at an initial pressure of P0=4 bar, and temperature of T0=400K. The gas goes through the following cycle. You can assume that all the processes are reversible. Spring 2023 PGE 326 a) Isothermal Expansion: The gas is brought into contact with a heat bath at TH=T0= 500K and is isothermally expanded until P1=3 bar. b) Adiabatic Expansion: The gas is decoupled from the bath and expanded adiabatically until P2=2 bar. c) Isothermal Compression: The gas is brought into contact with a heat bath at Tc= T2 and is isothermally compressed until P3=2.66 bar. d) Adiabatic Compression: The gas is decoupled from the bath and compressed adiabatically until P4=P0=4 bar. Samnle tahle. Note: For P,T, and V give the value after each step. For U,S,Q, and W give the value during the step (they are differences). For the total, only need to sum the last 4 columns
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


