Question: Please find the bottom operating line (only thing that I can't find ) = D17. A mixture of acetone and ethanol (acetone is more volatile)
Please find the bottom operating line (only thing that I can't find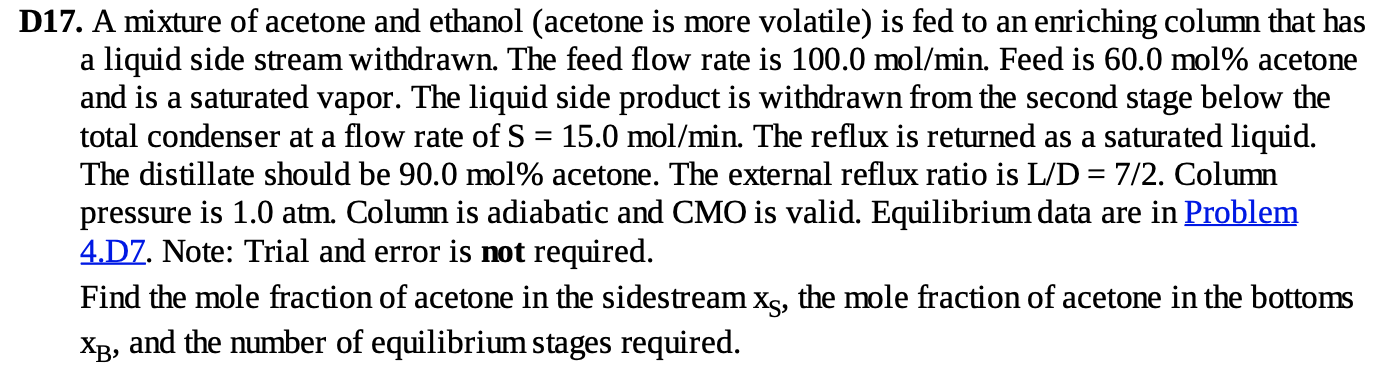
 )
)
= D17. A mixture of acetone and ethanol (acetone is more volatile) is fed to an enriching column that has a liquid side stream withdrawn. The feed flow rate is 100.0 mol/min. Feed is 60.0 mol% acetone and is a saturated vapor. The liquid side product is withdrawn from the second stage below the total condenser at a flow rate of S = 15.0 mol/min. The reflux is returned as a saturated liquid. The distillate should be 90.0 mol% acetone. The external reflux ratio is L/D = 7/2. Column pressure is 1.0 atm. Column is adiabatic and CMO is valid. Equilibrium data are in Problem 4.D7. Note: Trial and error is not required. Find the mole fraction of acetone in the sidestream Xs, the mole fraction of acetone in the bottoms Xp, and the number of equilibrium stages required. .10 .262 .15 .348 .20 -417 .25 478 .30 .524 .35 .566 .40 .605 .50 .674 .60 .739 .70 .802 .80 .865 .90 .929
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


