Question: please help with 1-8 using Beard Marianna J numbers . Due tomorrow at 1:00. 3 CENG 380 Test 3 3/18/2022 A continuous, steady-state distillation column
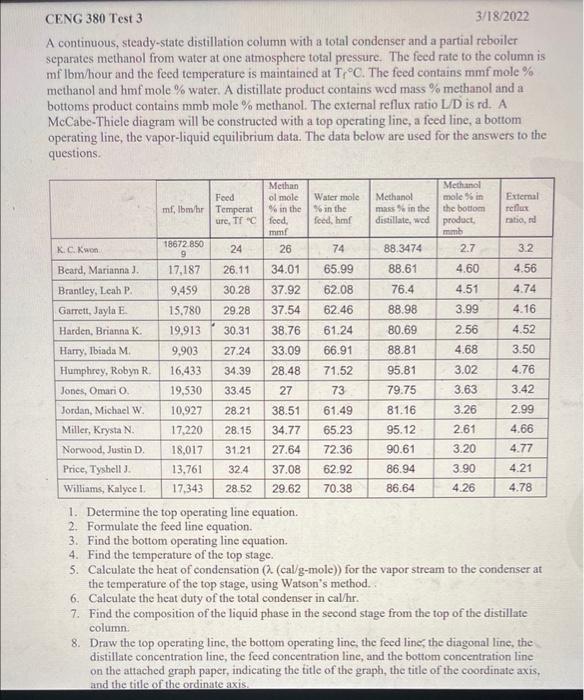
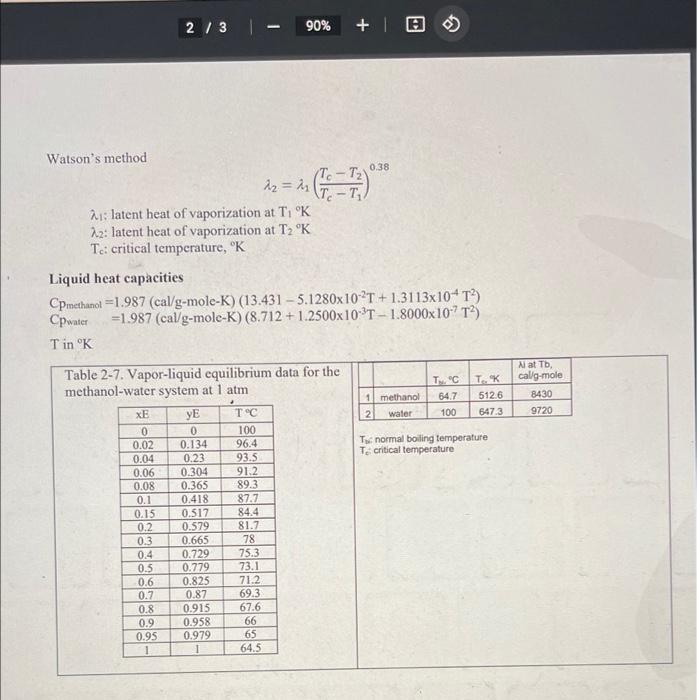
3 CENG 380 Test 3 3/18/2022 A continuous, steady-state distillation column with a total condenser and a partial reboiler separates methanol from water at one atmosphere total pressure. The feed rate to the column is mflbm/hour and the feed temperature is maintained at TrC. The feed contains mmf mole % methanol and hmf mole % water. A distillate product contains wed mass % methanol and a bottoms product contains mmb mole % methanol. The external reflux ratio L/D is rd. A McCabe-Thiele diagram will be constructed with a top operating line, a feed line, a bottom operating line, the vapor-liquid equilibrium data. The data below are used for the answers to the questions. 19.530 73 Methan Methanol Food ol mole Wafer mole Methanol mole in External mf, Ibm/hr Temperat % in the S6 in the mass in the the bottom reflux ure, TT Cloed, feed, hm distillate, wed product, ratio, ed mm mb 18672 850 K.C. Kwon 24 26 74 88.3474 2.7 3.2 9 Beard, Marianna). 17,187 26.11 34.01 65.99 88.61 4.60 4.56 Brantley, Leah P. 9.459 30.28 37.92 62.08 76.4 4.51 4.74 Garrett, Jayla E 15,780 29.28 37.54 62.46 88.98 3.99 4.16 Harden, Brianna K 19.913 30.31 38.76 61.24 80.69 2.56 4.52 Harry, Ibiada M 9.903 27.24 33.09 66.91 88.81 4.68 3.50 Humphrey, Robyn R 16,433 34.39 28.48 71.52 95.81 3.02 4.76 Jones, Omario 33.45 27 79.75 3.63 3.42 Jordan, Michael W. 10,927 28.21 38.51 61.49 81.16 3.26 2.99 Miller, Krysta N 17,220 28.15 34.77 65.23 95.12 2.61 4.66 Norwood, Justin D. 18,017 31.21 27.64 72.36 90.61 3.20 4.77 Price, Tyshell. 13,761 32.4 37.08 62.92 86.94 3.90 4.21 Williams, Kalyce ! 17.343 28.52 29.62 70.38 86.64 4.26 4.78 1. Determine the top operating line equation. 2. Formulate the feed line equation. 3. Find the bottom operating line equation. 4. Find the temperature of the top stage. 5. Calculate the heat of condensation (a (cal/g-mole)) for the vapor stream to the condenser at the temperature of the top stage, using Watson's method. 6. Calculate the heat duty of the total condenser in cal/hr. 7. Find the composition of the liquid phase in the second stage from the top of the distillate column. 8. Draw the top operating line, the bottom operating line, the feed line the diagonal line, the distillate concentration line, the feed concentration line, and the bottom concentration line on the attached graph paper, indicating the title of the graph, the title of the coordinate axis. and the title of the ordinate axis. 2/31 90% + O 0.38 Watson's method (To - Tz 22 = h T-T 21: latent heat of vaporization at TK 22: latent heat of vaporization at T2 K T.: critical temperature, K Liquid heat capacities Cpmethanol =1.987 (cal/g-mole-K) (13.431 - 5.1280x10 2T + 1.3113x10T Cpwater =1.987 (cal/g-mole-K) (8.712 +1.2500x102T - 1.8000x10-?T?) T in K TC 64.7 N at Tb, cal g-mole 8430 9720 T. K 5126 647.3 1 methanol 2 water 100 To normal boiling temperature T critical temperature 0.1 Table 2-7. Vapor-liquid equilibrium data for the methanol-water system at 1 atm XE y TC 0 0 100 0.02 0.134 96.4 0.04 0.23 93.5 0.06 0.304 91.2 0.08 0.365 89.3 0.418 87.7 0.15 0.517 84.4 0.2 0.579 81.7 0.3 0.665 78 0.4 0.729 75.3 0.779 73.1 0.6 0.825 71.2 0.7 0.87 69.3 0.8 0.915 67.6 0.9 0.958 66 0.95 0.979 65 1 1 64.5 lolololololo 818a 0.5 3/31 90% + 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


