Question: please full answer for good feed back. Q.3 (4.0 points) Assuming Raoult's law to be valid, prepare the following: a) Phase diagram (T-xy) at 90
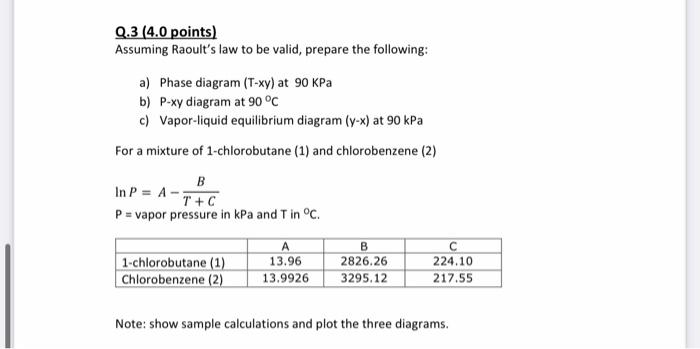
Q.3 (4.0 points) Assuming Raoult's law to be valid, prepare the following: a) Phase diagram (T-xy) at 90 KPa b) P-xy diagram at 90 C c) Vapor-liquid equilibrium diagram (9-x) at 90 kPa For a mixture of 1-chlorobutane (1) and chlorobenzene (2) B In P = A-T+C P = vapor pressure in kPa and T in C. 1-chlorobutane (1) Chlorobenzene (2) A 13.96 13.9926 B 2826.26 3295.12 224.10 217.55 Note: show sample calculations and plot the three diagrams
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


