Question: please help 1. Predict the ionic substance in each pair that has the greater melting point, and explain why: (a) AgCl or Zns (b) CrBr2
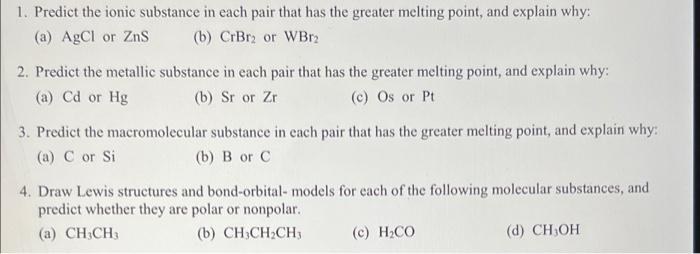
1. Predict the ionic substance in each pair that has the greater melting point, and explain why: (a) AgCl or Zns (b) CrBr2 or WBr2 2. Predict the metallic substance in each pair that has the greater melting point, and explain why: (a) Cd or Hg (b) Sr or Zr (c) Os or Pt 3. Predict the macromolecular substance in each pair that has the greater melting point, and explain why: (a) C or Si (b) B or C 4. Draw Lewis structures and bond-orbital-models for each of the following molecular substances, and predict whether they are polar or nonpolar. (a) CH:CH (b) CH3CH2CH (c) H2CO (d) CHOH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


