Question: Please help (a) Figure 1 represents the zinc-lead flotation process that is designed to accommodate a feed amount of about 100 kmol/ hour. The results
Please help
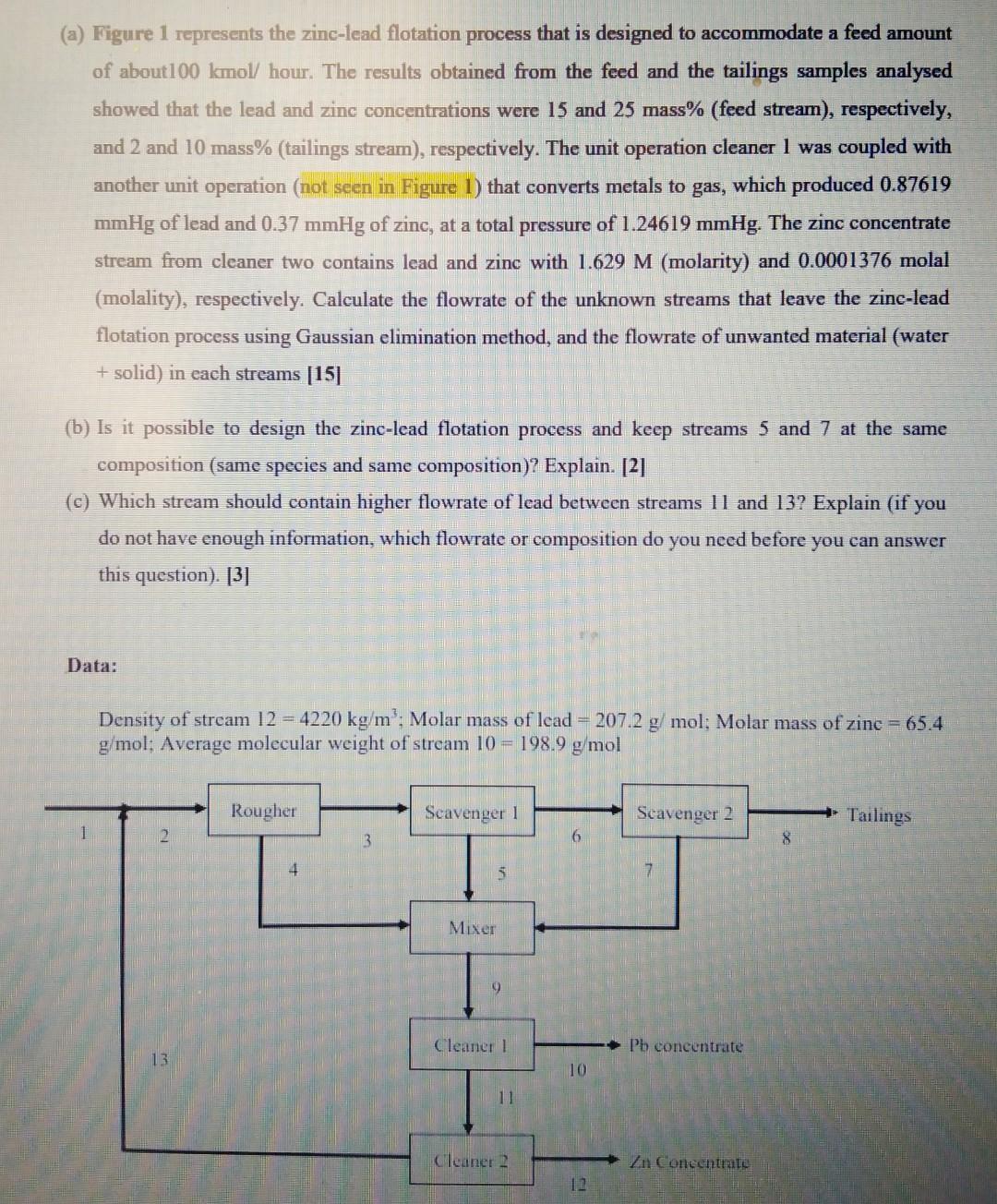
(a) Figure 1 represents the zinc-lead flotation process that is designed to accommodate a feed amount of about 100 kmol/ hour. The results obtained from the feed and the tailings samples analysed showed that the lead and zinc concentrations were 15 and 25 mass% (feed stream), respectively, and 2 and 10 mass% (tailings stream), respectively. The unit operation cleaner 1 was coupled with another unit operation (not seen in Figure 1) that converts metals to gas, which produced 0.87619 mmHg of lead and 0.37 mmHg of zinc, at a total pressure of 1.24619 mmHg. The zinc concentrate stream from cleaner two contains lead and zinc with 1.629 M (molarity) and 0.0001376 molal (molality), respectively. Calculate the flowrate of the unknown streams that leave the zinc-lead flotation process using Gaussian elimination method, and the flowrate of unwanted material (water + solid) in each streams [15] at the same (b) Is it possible to design the zinc-lead flotation process and keep streams 5 and composition (same species and same composition)? Explain. [2] (c) Which stream should contain higher flowrate of lead between streams 11 and 13? Explain (if you do not have enough information, which flowrate or composition do you need before you can answer this question). [3] Data: Density of stream 12 - 4220 kg/m': Molar mass of lead = 207.2 g/mol; Molar mass of zinc = 65.4 g/mol; Average molecular weight of stream 10 = 198.9 g/mol Rougher Scavenger 1 Scavenger 2 Tailings 3 4 5 7 Mixer Cleaner 1 Pb concentrate 13 10 11 Cleaner 2 Zn Concentrate 12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


