Question: please help! A solution contains 1.36102M silver nitrate and 1.11102M copper(II) acetate. Solid sodium hydroxide is added slowly to this mixture. What is the concentration
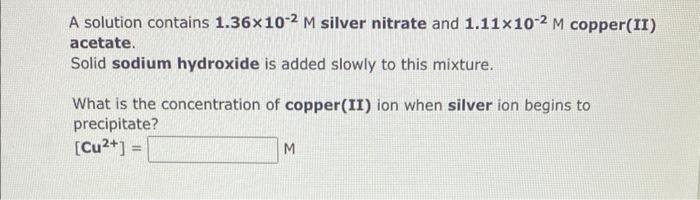
A solution contains 1.36102M silver nitrate and 1.11102M copper(II) acetate. Solid sodium hydroxide is added slowly to this mixture. What is the concentration of copper(II) ion when silver ion begins to precipitate? [Cu2+]= M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


