Question: Please help, and please try to explain. 6. The forward rate for the reaction Ce 4+ (aq) + Fe 2+ (aq) Ce 3+ (aq) +
Please help, and please try to explain.
6. The forward rate for the reaction Ce 4+ (aq) + Fe 2+ (aq) Ce 3+ (aq) + Fe 3+ (aq) is first order in [Ce 4+ ] and [Fe 2+ ], and second order overall. The rate constant k has been determined to be 1.00 x 10 3 L/mol s at 25 degrees C. In an experiment, 100.00 mL of 0.010 M Ce(SO 4 ) 2 solution is mixed rapidly with 100.00 mL 0f 0.010 M FeSO 4 solution and allowed to react. Calculate the concentration of Ce 4+ remaining after 2.0 seconds. (NOTE: You my assume that Ce 4+ and Fe 2+ are consumed at the same rate (1:1), so use the integrated second-order rate law expression in your calculation.)
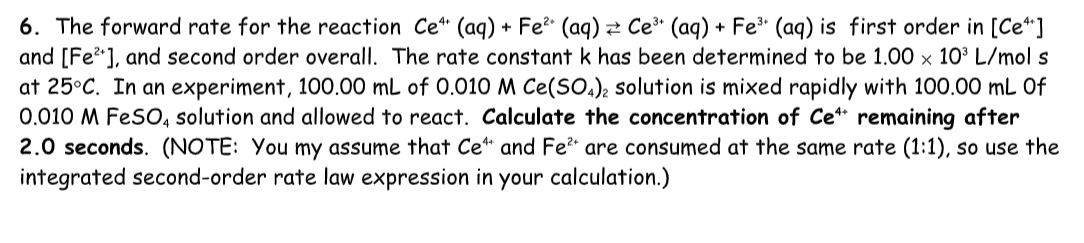
6. The forward rate for the reaction Ce4+(aq)+Fe2(aq)Ce3+(aq)+Fe3(aq) is first order in [Ce 4+] and [Fe2+], and second order overall. The rate constant k has been determined to be 1.00103L/mols at 25C. In an experiment, 100.00mL of 0.010MCe(SO4)2 solution is mixed rapidly with 100.00mL of 0.010MFeSO4 solution and allowed to react. Calculate the concentration of Ce4+ remaining after 2.0 seconds. (NOTE: You my assume that Ce4+ and Fe2+ are consumed at the same rate (1:1), so use the integrated second-order rate law expression in your calculation.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


