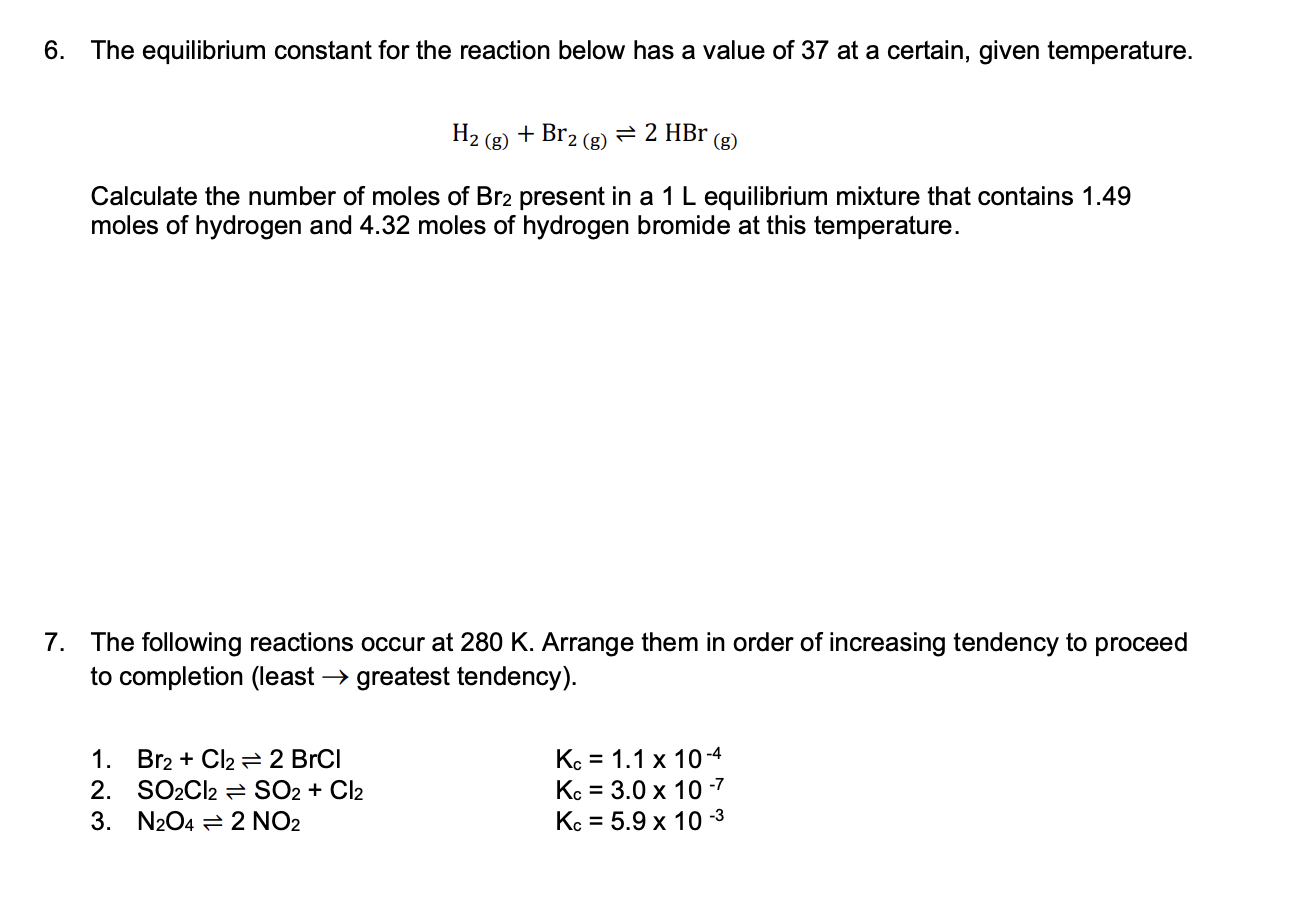
Question: please help and show work, thank you! The equilibrium constant for the reaction below has a value of 37 at a certain, given temperature. H2(g)+Br2(g)2HBr(g)
 please help and show work, thank you!
please help and show work, thank you!
The equilibrium constant for the reaction below has a value of 37 at a certain, given temperature. H2(g)+Br2(g)2HBr(g) Calculate the number of moles of Br2 present in a 1L equilibrium mixture that contains 1.49 moles of hydrogen and 4.32 moles of hydrogen bromide at this temperature. The following reactions occur at 280K. Arrange them in order of increasing tendency to proceed to completion (least greatest tendency). 1. Br2+Cl22BrCl Kc=1.1104 2. SO2Cl2SO2+Cl2 Kc=3.0107 3. N2O42NO2 Kc=5.9103
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


