Question: please help and solve for all blanks In a study of the rearrangement of ammonium cyanate to urea in aqueous solution at 50C NH4NCO(aq)(NH2)2CO(aq) the
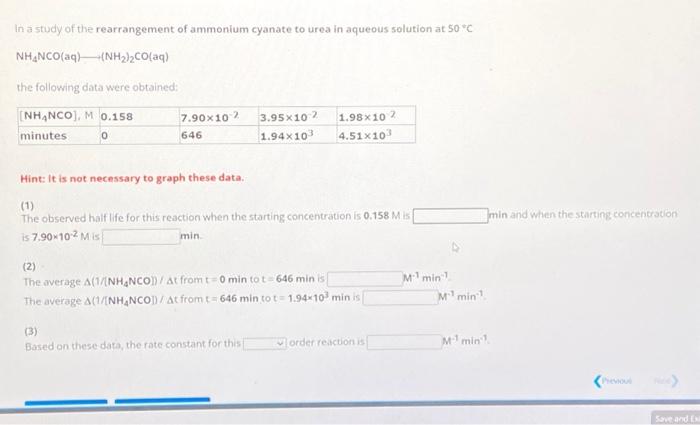
In a study of the rearrangement of ammonium cyanate to urea in aqueous solution at 50C NH4NCO(aq)(NH2)2CO(aq) the following data were obtained: Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.158M is min and when the starting concentration is 7.90102M is min. (2) The average A(1/[NH4NCO)//t from t=0mintot=646min is M1min1 The average (1/[NH4NCO])/ from t=646min to t=1.94103min is M1min1 (3) Based on these data, the rate constant for this order reaction is M1min1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


