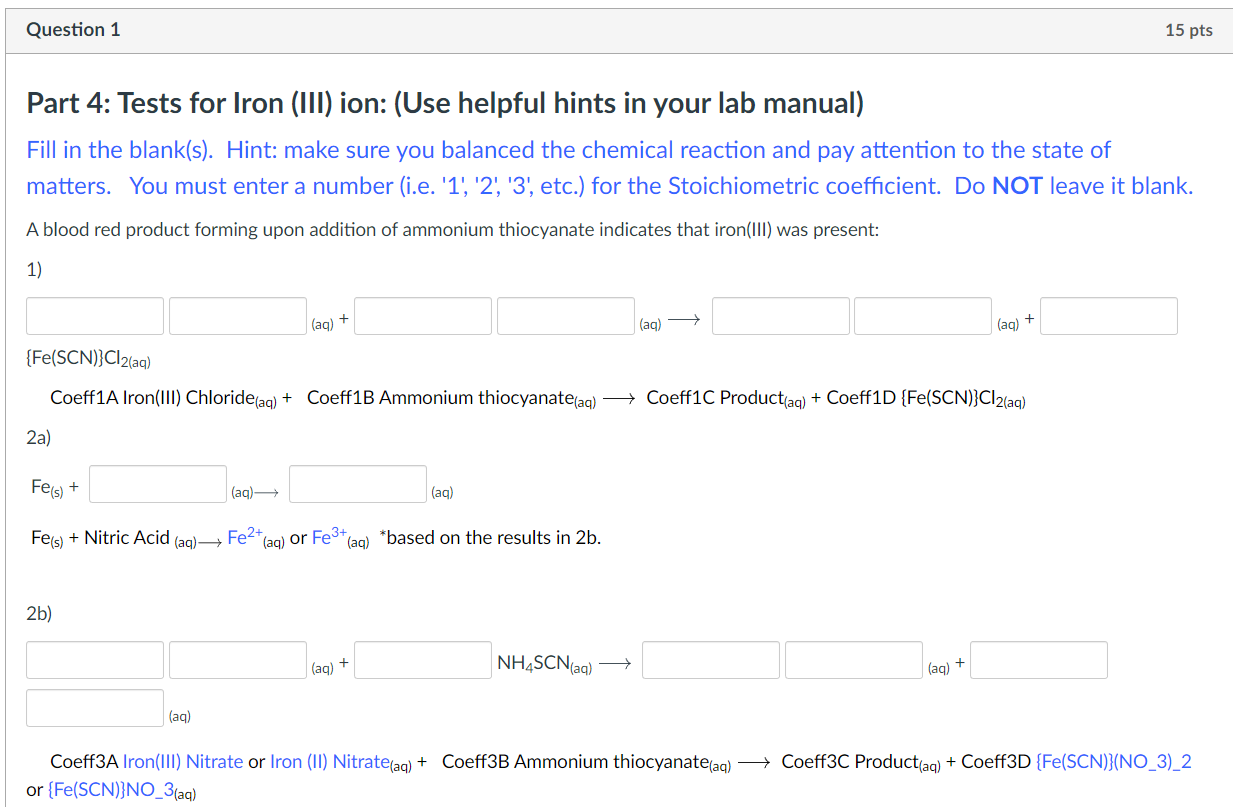
Question: Please help and use the formatting below. Thanks. Part 4: Tests for Iron (III) ion: (Use helpful hints in your lab manual) Fill in the
 Please help and use the formatting below. Thanks.
Please help and use the formatting below. Thanks.
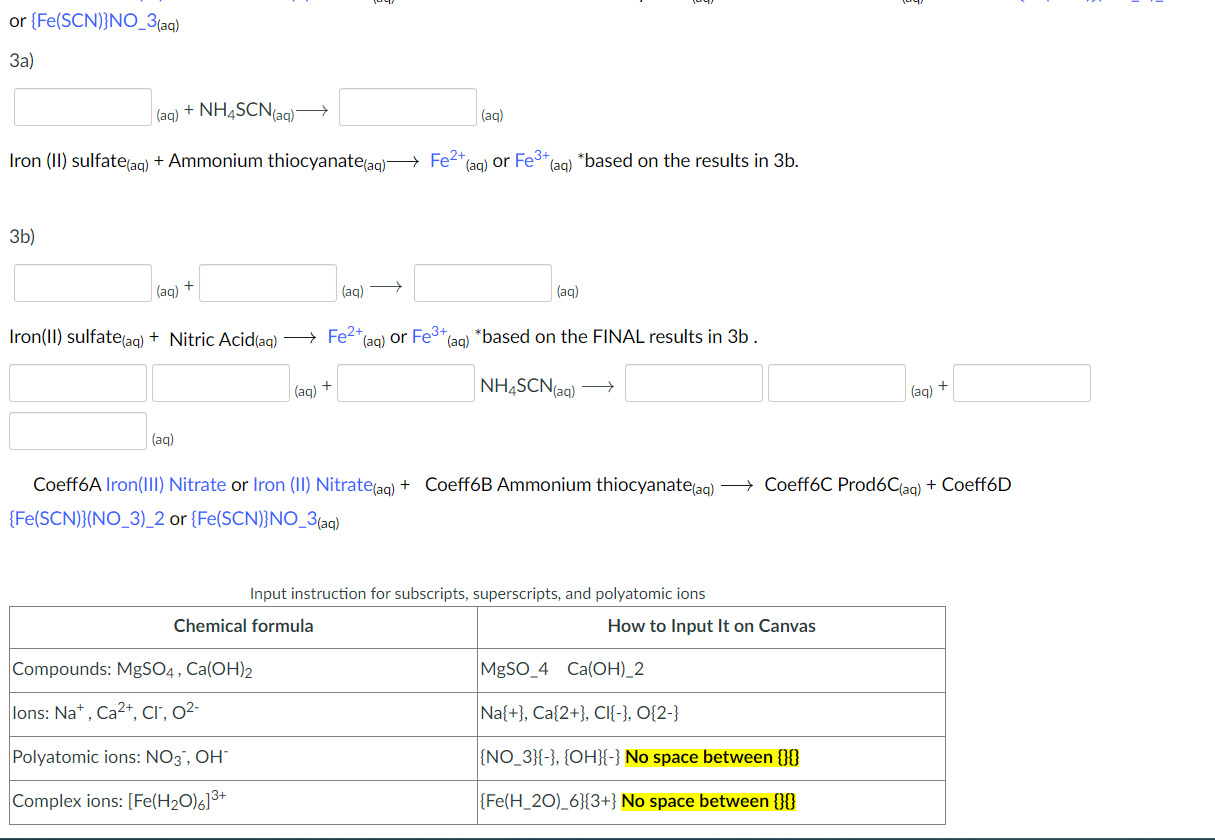
Part 4: Tests for Iron (III) ion: (Use helpful hints in your lab manual) Fill in the blank(s). Hint: make sure you balanced the chemical reaction and pay attention to the state of matters. You must enter a number (i.e. '1', '2', '3', etc.) for the Stoichiometric coefficient. Do NOT leave it blank. A blood red product forming upon addition of ammonium thiocyanate indicates that iron(III) was present: 1) or {Fe(SCN)}NO(aq) 3a) (aq)+NH4SCN(aq) 3b) (aq)+(aq) (aq)+NH4SCN(aq)(aq)+ (aq) {Fe(SCN)}(NO3)2 or {Fe(SCN)}NO3(aq) Input instruction for subscripts, superscripts, and polyatomic ions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


