Question: Please help answer all parts to the question! Due tonight A. Determination of Initial Concentrations Initial volume of NaOH. Final volume of NaOH : 38.00mL
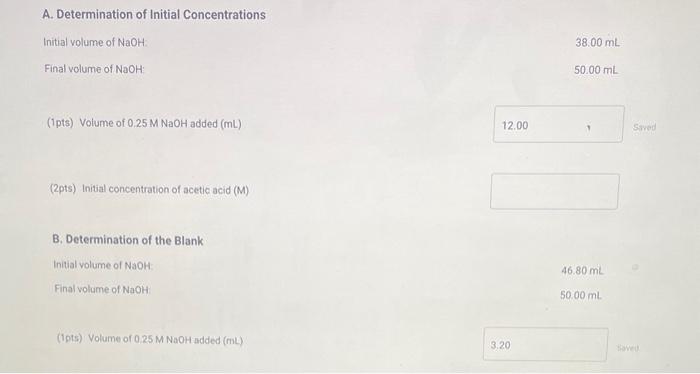
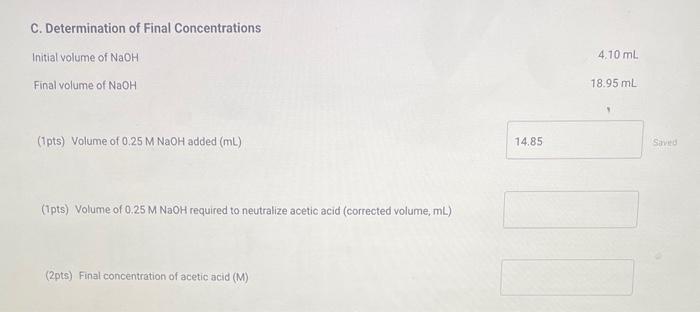
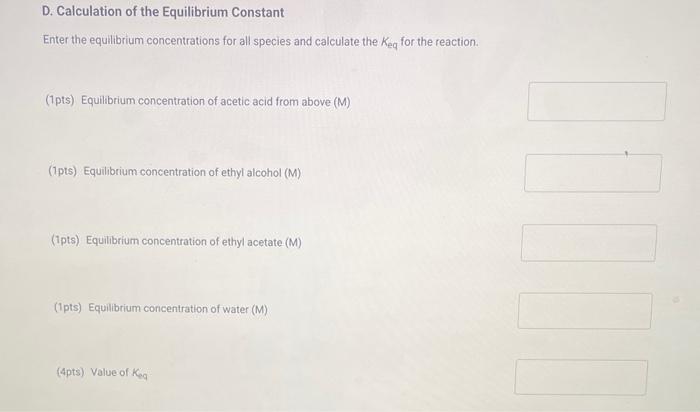
A. Determination of Initial Concentrations Initial volume of NaOH. Final volume of NaOH : 38.00mL 50.00mL (1pts) Volume of 0.25MNaOH added (mL) (2pts) Initial concentration of acetic acid (M) B. Determination of the Blank Initial volume of NaOH : Final volume of NaOH 46.80mL50.00mL (1pts) Volume of 0.25MNaOH added (mL) C. Determination of Final Concentrations Initial volume of NaOH Final volume of NaOH 18.95mL (1pts) Volume of 0.25MNaOH added ( mL) (1pts) Volume of 0.25MNaOH required to neutralize acetic acid (corrected volume, mL ) (2pts) Final concentration of acetic acid (M) D. Calculation of the Equilibrium Constant Enter the equilibrium concentrations for all species and calculate the Keq for the reaction. (1pts) Equilibrium concentration of acetic acid from above (M) (1pts) Equilbrium concentration of ethyl alcohol (M) (1pts) Equilibrium concentration of ethyl acetate (M) (1pts) Equilibrium concentration of water ( M ) (4pts) Value of Ke
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


