Question: PLEASE HELP ANSWER ALL Q11. Why couldn't carbon dioxide have a Lewis Structure more resembling the carbon tetrachloride? In other words, why are the bonds




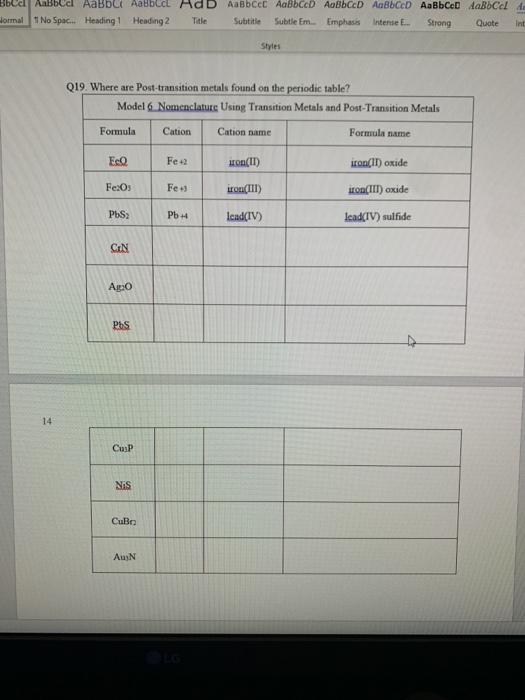
Q11. Why couldn't carbon dioxide have a Lewis Structure more resembling the carbon tetrachloride? In other words, why are the bonds between C and O not single bonds with each oxygen having three lone pair? (There are two reasons.) 15 I Q6. For some elements, there is no Greek prefix. What is the number then? Q12. What do the structures with the same number of electron groups have in common? Q14 How does the sum of the number of lone pairs and bonded atoms compare to the number of electron groups around the central atom? l t l di Hormal 1 No Space Heading 1 Heading 2 Title Subtitle Subtle Em Emphasis intense E... Strong Quote Styles Q19. Where are Post-transition metals found on the periodic table? Model 6 Nomenclature Using Transition Metals and Post-Transition Metals Formula Cation Cation name Formula name EQ Fe. Hon(IT) iron(II)oxide Fe2O3 Fe) iron(III) uro(III)oxide PbS PbH lead(IV) lead(TV) sulfide CIN Axo Phs 14 Cup Nis Culo AuN
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


