Question: please help answer correctly. thank you Handout: Interpreting Kinetics Data Two experiments were done to study the reaction AB. Experiment I started with an initial
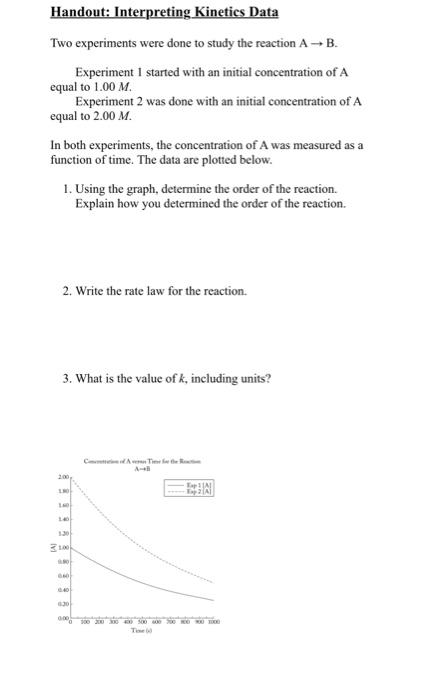
Handout: Interpreting Kinetics Data Two experiments were done to study the reaction AB. Experiment I started with an initial concentration of A equal to 1.00 M Experiment 2 was done with an initial concentration of A equal to 2.00 M. In both experiments, the concentration of A was measured as a function of time. The data are plotted below. 1. Using the graph, determine the order of the reaction. Explain how you determined the order of the reaction. 2. Write the rate law for the reaction. 3. What is the value of k, including units? 1.30 100 0.30 0.00
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


