Question: please help answer Select the statement that is true regarding a reaction system at equilbrium. A. The number of collisions per unit time between reactants
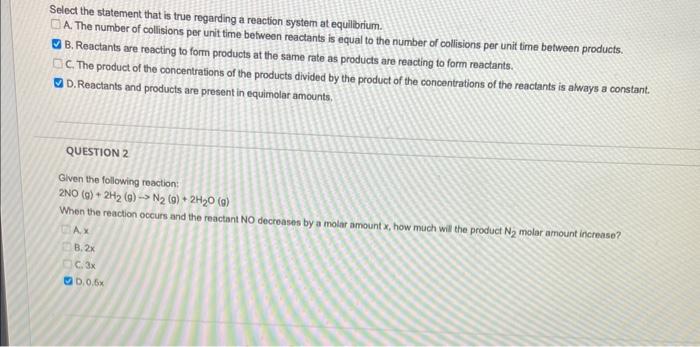
Select the statement that is true regarding a reaction system at equilbrium. A. The number of collisions per unit time between reactants is equal to the number of collisions per unit time between products. B. Reactants are reacting to form products at the same rate as products are reacting to form reactants. C. The product of the concentrations of the products divided by the product of the concentrations of the reactants is always a constant. D. Reactants and products are present in equimolar amounts. QUESTION 2 Given the following reaction: 2NO(g)+2H2(g)N2(g)+2H2O(g) When the reaction occurs and the reactant NO decroases by a molar amount x, how much wil the product N2 molar amount increase? A.x B. 2x C. 3x b. 0.6x
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


