Question: PLEASE HELP ASAP 1. Based on your calculated extinction coefficient, estimate the concentration of an unknown CuSO4 solution with an absorbance of 0.65 ? SerialDilutions


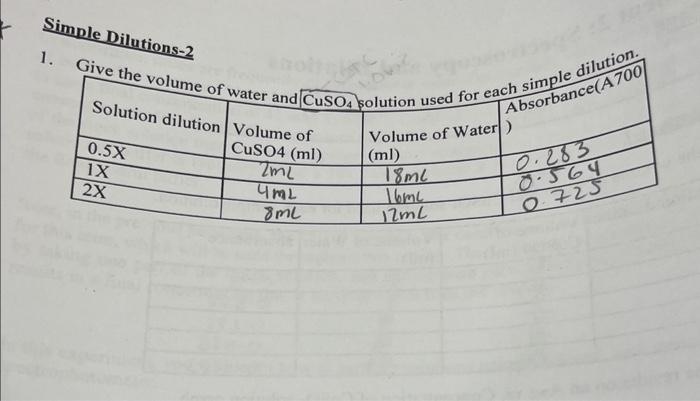

1. Based on your calculated extinction coefficient, estimate the concentration of an unknown CuSO4 solution with an absorbance of 0.65 ? SerialDilutions Tolal volume =10mL 1. Describe how each serial dilution was prepared. Dilutions-2 Give the volume of was. 1. Give the volume of water and CuSO4 solution used for each. 4. Plot the results on an A700 vs. Concentration of CuSO4 solution graph. 1. Based on your calculated extinction coefficient, estimate the concentration of an unknown CuSO4 solution with an absorbance of 0.65 ? SerialDilutions Tolal volume =10mL 1. Describe how each serial dilution was prepared. Dilutions-2 Give the volume of was. 1. Give the volume of water and CuSO4 solution used for each. 4. Plot the results on an A700 vs. Concentration of CuSO4 solution graph
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


