Question: please help asap A student dissolves 14. 8 of urea ((NH2)2CO) in 275. mL of a solvent with a density of 1.10 gint. The stiudent
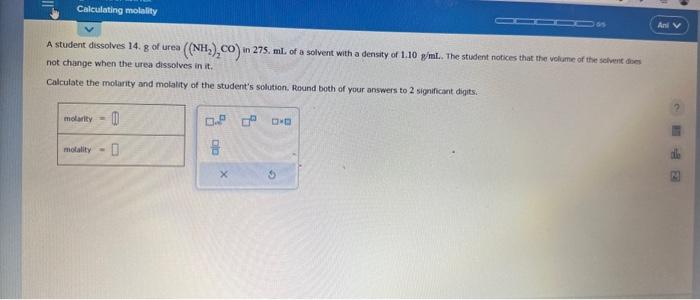
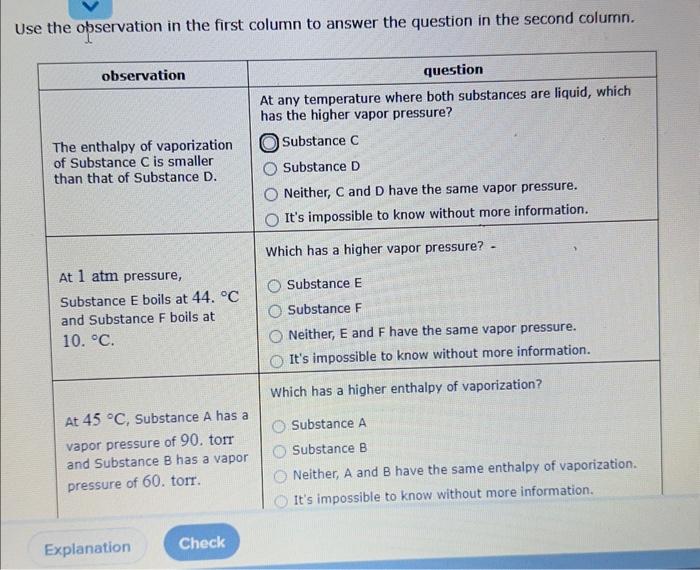
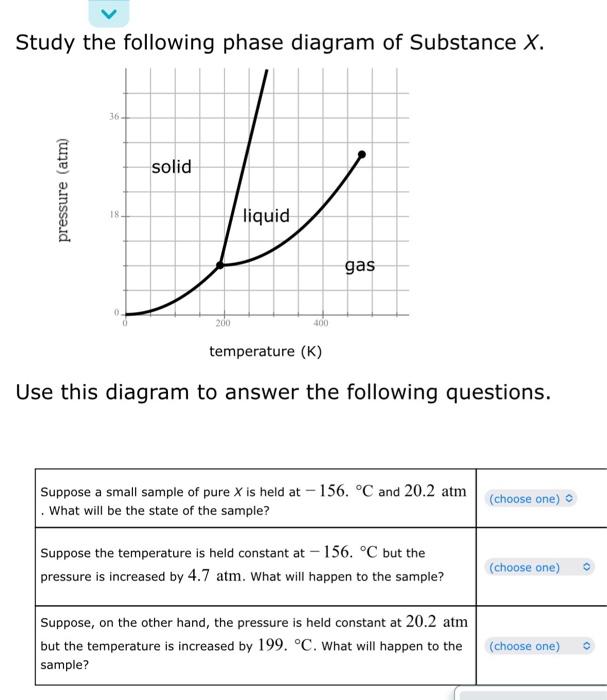
A student dissolves 14. 8 of urea ((NH2)2CO) in 275. mL of a solvent with a density of 1.10 gint. The stiudent notices that the volume of the selvent does not change when the urea dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits. se the observation in the first column to answer the question in the second column. \begin{tabular}{|l|} \hline \multicolumn{1}{|c|}{ observation } \\ \hline TheenthalpyofvaporizationofSubstanceCissmallerthanthatofSubstanceD. \\ At 1 atm pressure, \\ Substance E boils at 44 . C \\ and Substance F boils at \\ 10.C. \\ \hline \\ At 45 . C, Substance A has a \\ vapor pressure of 90 , torr \\ and Substance B has a vapor \\ pressure of 60. torr. \end{tabular} Study the following phase diagram of Substance X. Use this diagram to answer the following questions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


