Question: PLEASE HELP ASAP The two resonance structures for Structure 1 are: and The resonance structure that contributes the most to the hybrid is: If the
PLEASE HELP ASAP 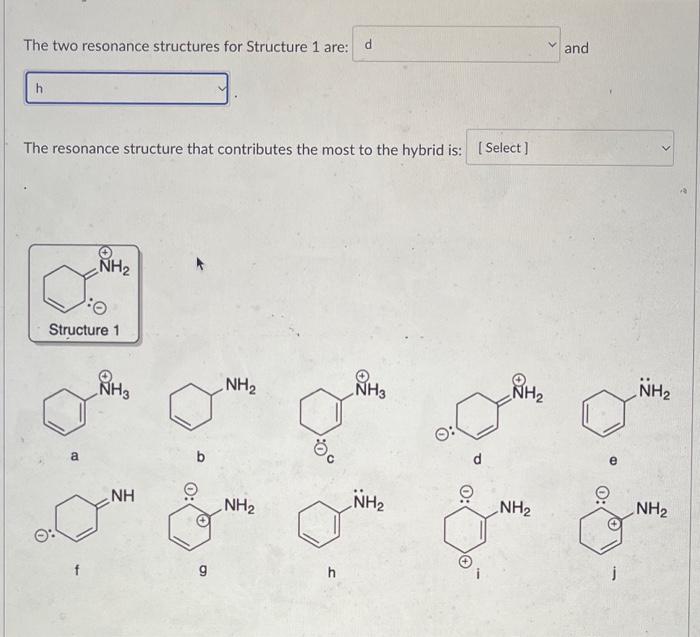
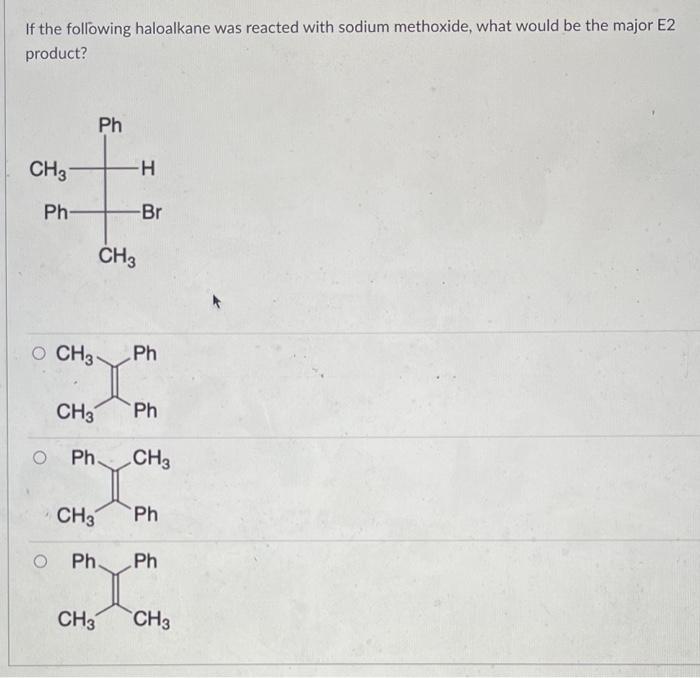
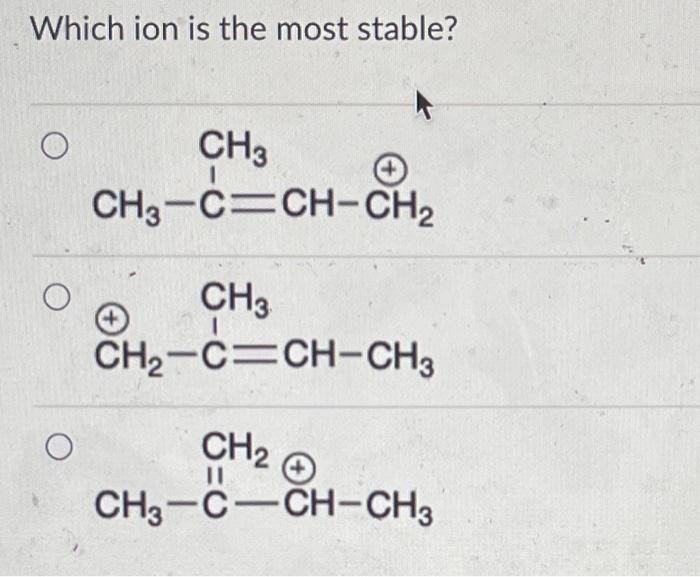



The two resonance structures for Structure 1 are: and The resonance structure that contributes the most to the hybrid is: If the following haloalkane was reacted with sodium methoxide, what would be the major E2 product? Which ion is the most stable
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


