Question: PLEASE HELP ASAP WILL LIKE!! ANSWER ALL QUESTIONS 1. In general chemistry you probably learned that HCl,HBr, and HI are all strong acids with no
PLEASE HELP ASAP WILL LIKE!! ANSWER ALL QUESTIONS 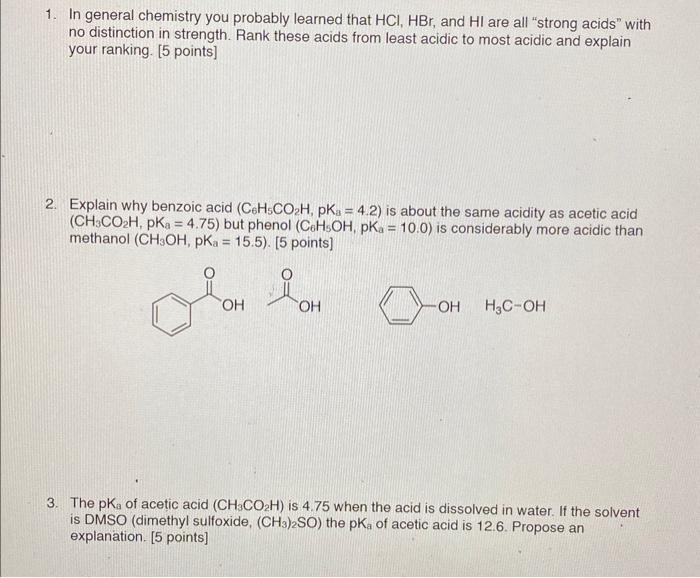

1. In general chemistry you probably learned that HCl,HBr, and HI are all "strong acids" with no distinction in strength. Rank these acids from least acidic to most acidic and explain your ranking. [5 points] 2. Explain why benzoic acid (C6H5CO2H,pKa=4.2) is about the same acidity as acetic acid (CH3CO2H,pKa=4.75) but phenol (C6H5OH,pKa=10.0) is considerably more acidic than methanol (CH3OH,pKaa=15.5). [5 points] H3COH 3. The pKa of acetic acid (CH3CO2H) is 4.75 when the acid is dissolved in water. If the solvent is DMSO (dimethyl sulfoxide, (CH3)2SO ) the pKa of acetic acid is 12.6. Propose an explanation. [5 points]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


