Question: Please help before 11:59 pm! Explanation and steps. Consider the reaction: N2(S)+O2(8)2NO(.3) The reaction is carried out in a tempersture that you have Kc=0.055. The
Please help before 11:59 pm! Explanation and steps.
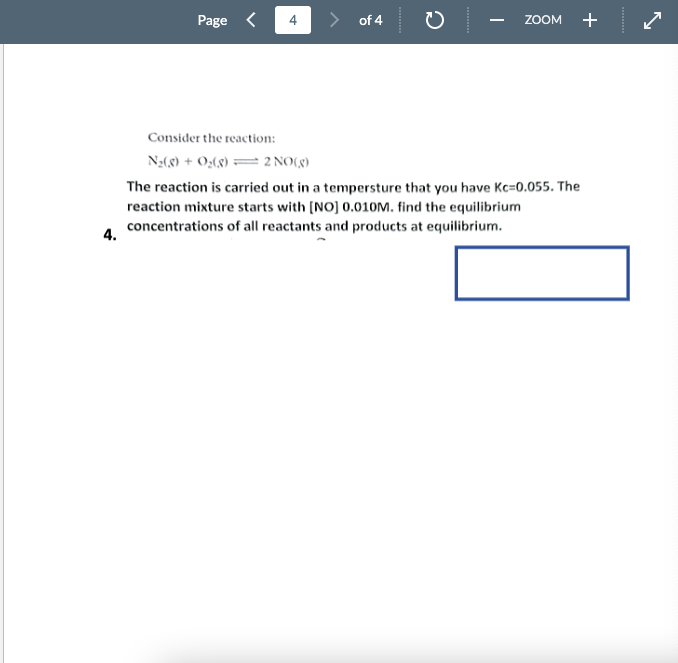
Consider the reaction: N2(S)+O2(8)2NO(.3) The reaction is carried out in a tempersture that you have Kc=0.055. The reaction mixture starts with [NO] 0.010M. find the equilibrium concentrations of all reactants and products at equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


