Question: Please help before 11:59 pm! Explanation and steps. The equilibrium constant is given for one of the reactions below. Determine the value of missing equilibrium
Please help before 11:59 pm! Explanation and steps.
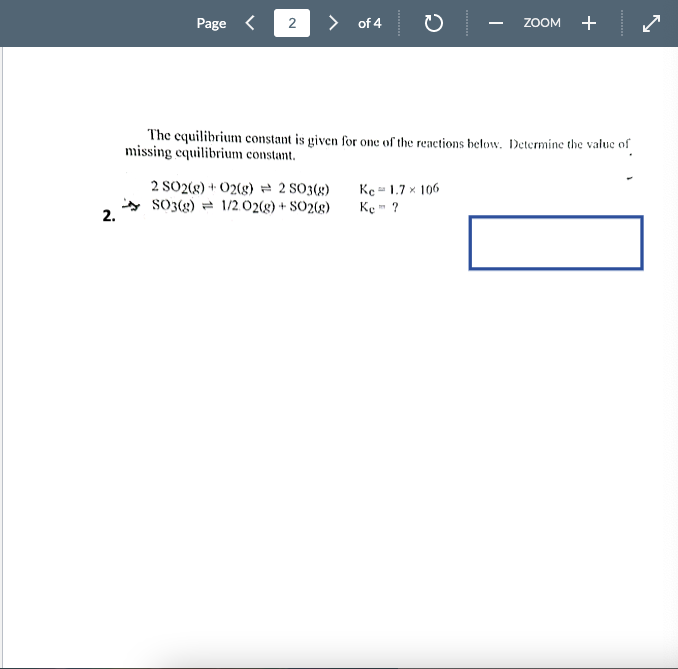
The equilibrium constant is given for one of the reactions below. Determine the value of missing equilibrium constant. 2SO2(g)+O2(g)2SO3(g)Kc=1.7106 2. SO3(g)1/2O2(g)+SO2(g)Ke=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


