Question: please help! Consider a hypothetical chemical reaction: A+ BC+D (In this equation A, B, C and D stand for some unknown chemical formulas.) Here is
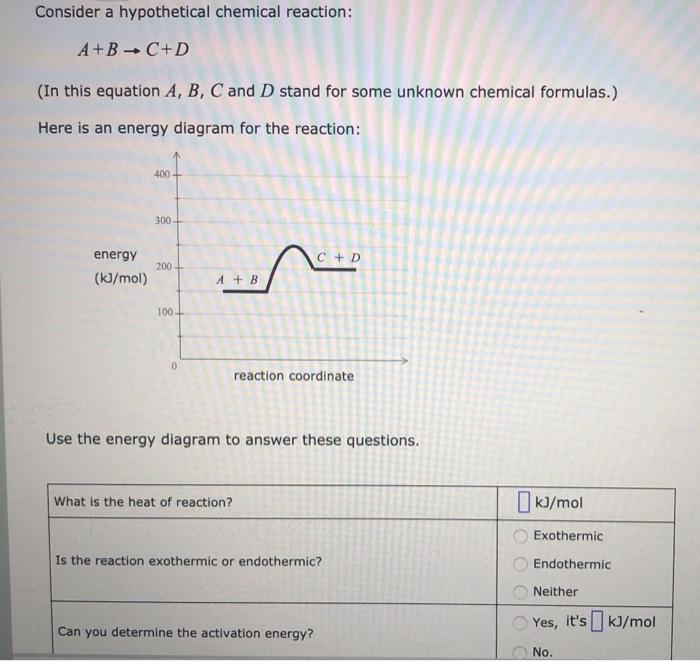
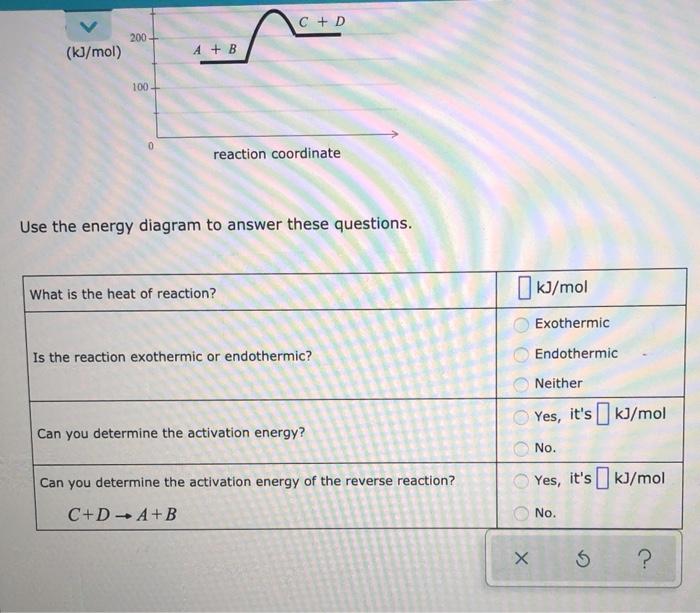
Consider a hypothetical chemical reaction: A+ BC+D (In this equation A, B, C and D stand for some unknown chemical formulas.) Here is an energy diagram for the reaction: 400 300 C+D energy (kJ/mol) 200 A + B 100+ 0 reaction coordinate Use the energy diagram to answer these questions. What is the heat of reaction? kJ/mol Exothermic Is the reaction exothermic or endothermic? Endothermic Neither Yes, it's ] kJ/mol Can you determine the activation energy? No. C + D 200 (kJ/mol) A + B 100 0 reaction coordinate Use the energy diagram to answer these questions. What is the heat of reaction? (kJ/mol Exothermic Is the reaction exothermic or endothermic? OOOOO Endothermic Neither Yes, it's [] kJ/mol Can you determine the activation energy? No. Can you determine the activation energy of the reverse reaction? Yes, it's kJ/mol C+ DA+B No. 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


