Question: please help data: (3) - Use the method of initial rates to solve the rate law expression. Remember, the reaction orders are usually whole numbers

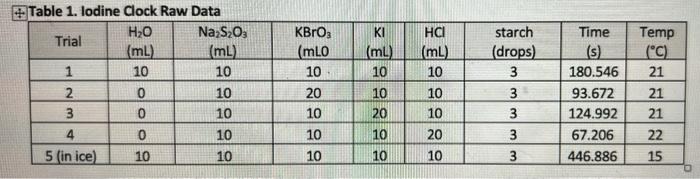
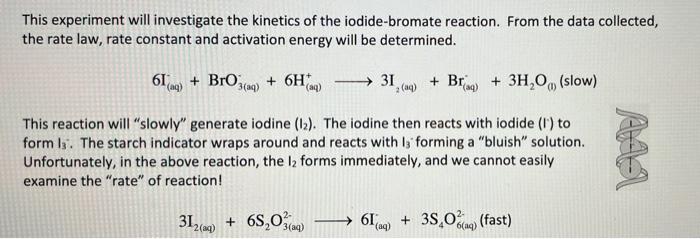
(3) - Use the method of initial rates to solve the rate law expression. Remember, the reaction orders are "usually" whole numbers but, might be y or 1/3 if the reaction is complicated! Make sure you report the order of each reagent and the overall "order of reaction". (4) - Write the correct rate law for the reaction. Table 1. Iodine Clock Raw Data H2O Na S2O3 Trial (mL) ImL) 1 10 10 2 0 10 3 0 10 4 0 10 5 (in ice) 10 10 KBrO; (mLO 10 20 KI (ml) 10 10 20 HCI (mL) 10 10 10 20 10 starch (drops) 3 3 3 3 3 Time (s) 180.546 93.672 124.992 67.206 446.886 Temp (C) 21 21 21 22 15 10 10 10 10 mlm 10 This experiment will investigate the kinetics of the iodide-bromate reaction. From the data collected, the rate law, rate constant and activation energy will be determined. 61m) + Bro + 6H 3(aq) (aq) 31 (aq) + Brag) + 3H,0, (slow) () () This reaction will "slowly" generate iodine (12). The iodine then reacts with iodide (1) to form 13. The starch indicator wraps around and reacts with is forming a "bluish" solution. Unfortunately, in the above reaction, the la forms immediately, and we cannot easily examine the "rate" of reaction! 312(aq) + 68,03100 619) + 38,0%(fast) 6(1) (3) - Use the method of initial rates to solve the rate law expression. Remember, the reaction orders are "usually" whole numbers but, might be y or 1/3 if the reaction is complicated! Make sure you report the order of each reagent and the overall "order of reaction". (4) - Write the correct rate law for the reaction. Table 1. Iodine Clock Raw Data H2O Na S2O3 Trial (mL) ImL) 1 10 10 2 0 10 3 0 10 4 0 10 5 (in ice) 10 10 KBrO; (mLO 10 20 KI (ml) 10 10 20 HCI (mL) 10 10 10 20 10 starch (drops) 3 3 3 3 3 Time (s) 180.546 93.672 124.992 67.206 446.886 Temp (C) 21 21 21 22 15 10 10 10 10 mlm 10 This experiment will investigate the kinetics of the iodide-bromate reaction. From the data collected, the rate law, rate constant and activation energy will be determined. 61m) + Bro + 6H 3(aq) (aq) 31 (aq) + Brag) + 3H,0, (slow) () () This reaction will "slowly" generate iodine (12). The iodine then reacts with iodide (1) to form 13. The starch indicator wraps around and reacts with is forming a "bluish" solution. Unfortunately, in the above reaction, the la forms immediately, and we cannot easily examine the "rate" of reaction! 312(aq) + 68,03100 619) + 38,0%(fast) 6(1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


