Question: please help Equilibrium constants at 25C are listed in the table below. Part A What is the molar solubility of marble (i.e., [Ca2+] in a
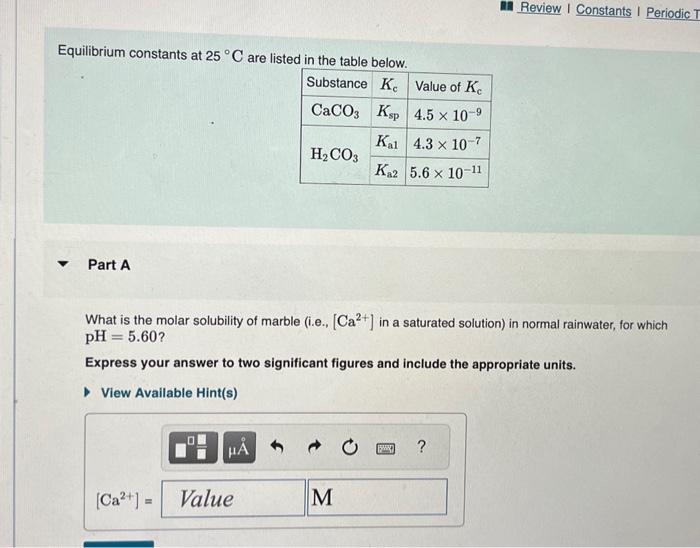
![below. Part A What is the molar solubility of marble (i.e., [Ca2+]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8f0e015c2d_62366f8f0dfbdc60.jpg)
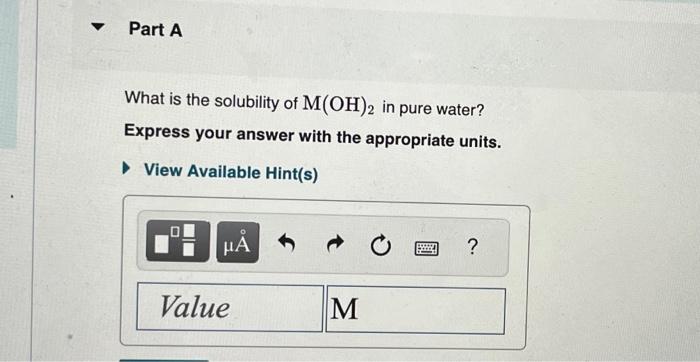
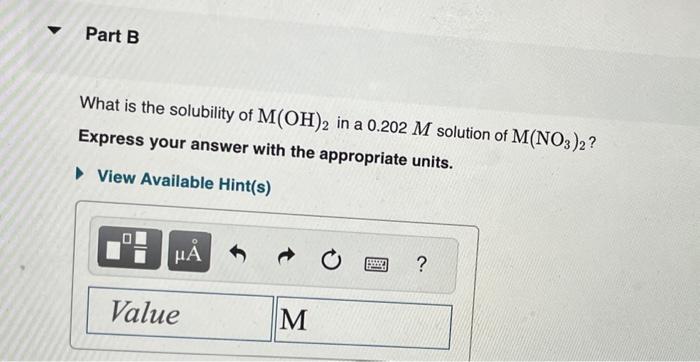
Equilibrium constants at 25C are listed in the table below. Part A What is the molar solubility of marble (i.e., [Ca2+] in a saturated solution) in normal rainwater, for which pH=5.60? Express your answer to two significant figures and include the appropriate units. The generic metal hydroxide M(OH)2 has Ksp=3.251012. (NOTE: In this particular problem, because of the magnitude of the Ksp and the stoichiometry of the compound, the contribution of OHfrom water can be ignored. However, this may not always be the case.) What is the solubility of M(OH)2 in pure water? Express your answer with the appropriate units. What is the solubility of M(OH)2 in a 0.202M solution of M(NO3)2 ? Express your answer with the appropriate units. View Available Hint(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


