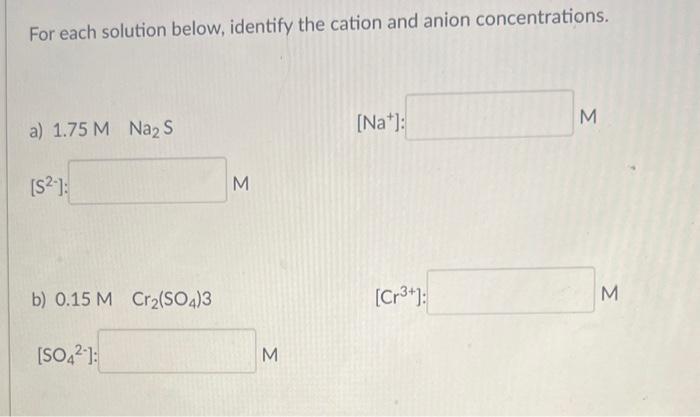
Question: please help For each solution below, identify the cation and anion concentrations. a) 1.75MNaNa2S [Na+] M [S2] M b) 0.15MCr(CO4)3 [Cr3+] M [SO42] : M
![a) 1.75MNaNa2S [Na+] M [S2] M b) 0.15MCr(CO4)3 [Cr3+] M [SO42] :](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d35ecc20b_07066f8d35e817b8.jpg)
For each solution below, identify the cation and anion concentrations. a) 1.75MNaNa2S [Na+] M [S2] M b) 0.15MCr(CO4)3 [Cr3+] M [SO42] : M What volume (in mL ) of 0.225MK3PO4 solution is required to completely react with 134mL of 0.0112MNiCl2, as shown below: 2K3PO4(aq)+3NiCl2(aq)Ni3(PO4)2(s)+6KCl(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


