Question: please help Ghemical cquilbrium exists when the formard reaction rate is equal to the reverue reaction ratr whim equilerium is: reached, there is no net
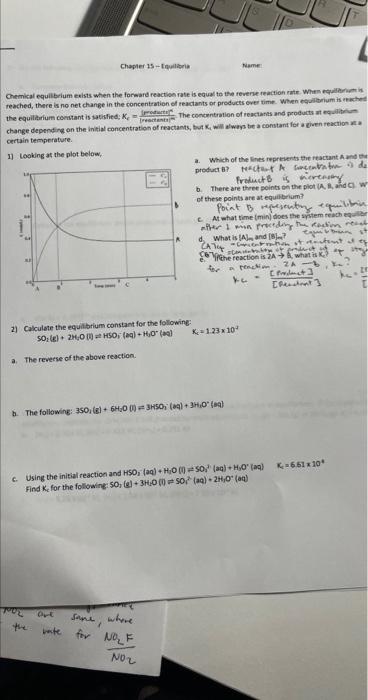



Ghemical cquilbrium exists when the formard reaction rate is equal to the reverue reaction ratr whim equilerium is: reached, there is no net change in the concentration of reactants or products ever time. When equilbrium is ukiched change depending on the initial concentration of reactants, but K4 will alweys be a constant fer a civen reaction at a certain temperature. 1) taoking at the plot below. a. Which of the lines reprieients the reactant A and ter freducts is ierencery b. There are theee points ea the piot (A, li, and C) W of these points are at equilbriam? pociat B wequewatons equabeis. c. At what time (min) does the syitem teach eqmilter 1 wis froerding the restion reint d. What is [A]m and [B]-7? C67 forthe feaction is 2A B. what is ke ? at it. ter in tenckine 2kb, the? 2) Calculate the equilibrium constant for the following: SO2(E)+2H4O(D)=HSO3(aq)+H1O(as)KC=1.2310+ a. The reverse of the above reaction. b. The following; 35O4(g)+6H2O(D)=3H5Oh(aa)+3H,O(Baa] c. Using the initial reaction and HSO1(aq)+H1O(0)NO32 (aq) +HyO2(ag)Kc=6.61104 Find Kc for the followinki 50,(9)+3H+0(0)=5002(aa)+2t(g+(aad) a. Complete the chart below: b. Cakculate the equilibrium partial pressures of eact gat (line Eabove): Uve them t find 6 . c. Use Xn=K4 (ldinh and compare is to the Ko form part b. 4) Use the equilibrium quotient, , to detemine it the following reactions are at equilbrium. it the seaction is net a a. [N3]=0.384M,[HU]=0.425M,[NHH3]=0.251M b. [N3]=5.25102M,[HA]=1.48102M,[NYt]=7.10104M vi. NO2 owt sines, where Find the equilibrium concentrations of each component for the following that occuri at high temgeratures N,(e)+O2(g)=2NO (e) x1=4.5101 3. When {N2}=0.350M and {O},=0.450M. There is no NO in the sydtem initially. b. When [N3)=0.200M,[O4]=0.200M, and (NO]1=0.100M. the chatelier's Principle to indicate the direction of the shift. Exalain wour m ramaning for choosing the 6 mectien fie (s)+3Cl3(B)2FeCl(s)AH=343Wd weasing the pressure of C2 in the spiem. pecreasing the temperature from 50C to 10C. c. Adding a small amount of H4 (g) to the system. H4(g) will react with the C4 (s). Aswme charges to pee sead pressure are negligible. d. Changing the reaction vessel volume from 5.0L to 7.0L e. Doubling the amount of iron metal in the system
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


