Question: please help Given that the initial rate constant is 0.0150s1 at an initial temperature of 26C, what would the rate constant be at a temperature

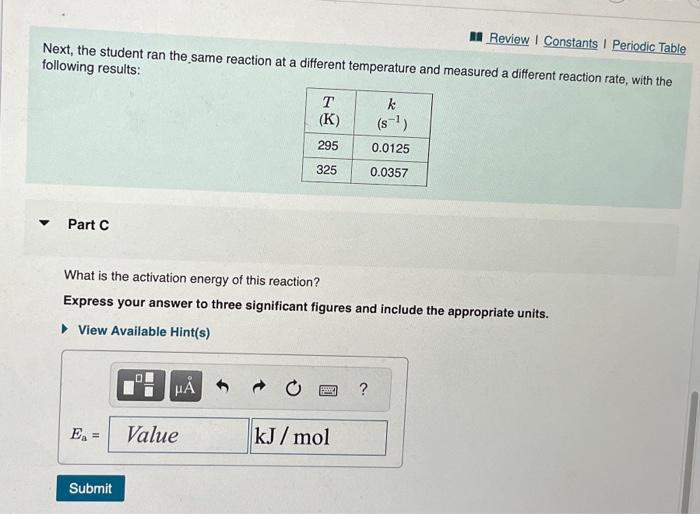
Given that the initial rate constant is 0.0150s1 at an initial temperature of 26C, what would the rate constant be at a temperature of 200.C for the same reaction described in Part A? Express your answer with the appropriate units. Next, the student ran the same reaction at a different temperature and measured a different reaction rate, with the following results: Part C What is the activation energy of this reaction? Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


