Question: Please help, I will upvote, thank you so much! A certain weak acid, HA, has a Ka value of 9.4107. Percent ionization for a weak
Please help, I will upvote, thank you so much!
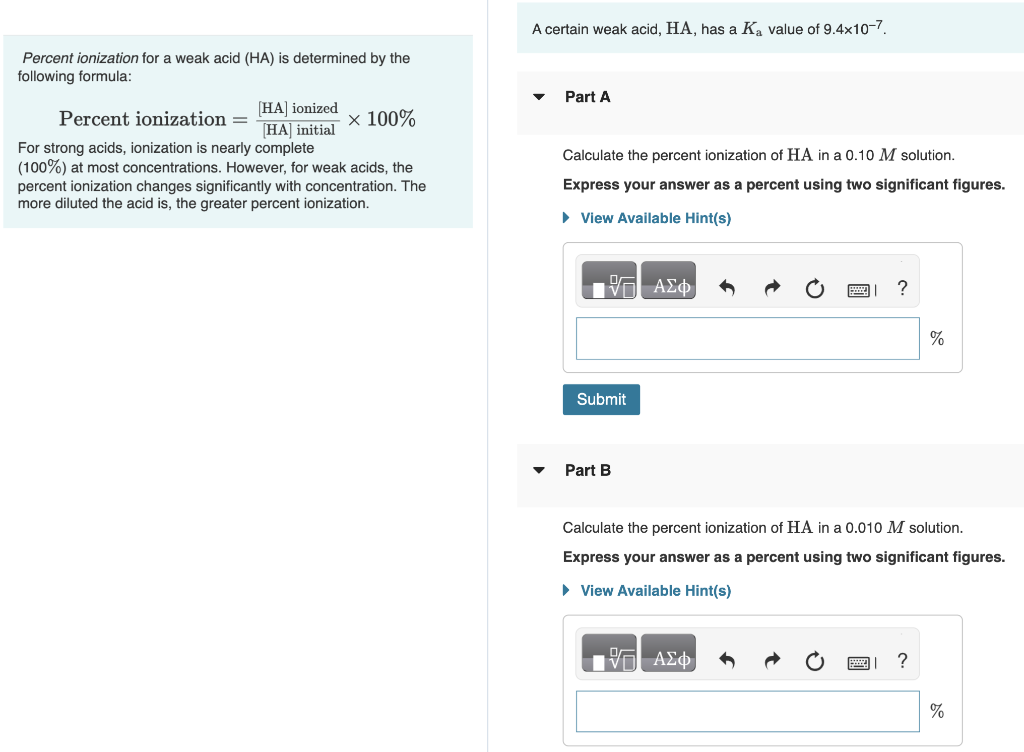
A certain weak acid, HA, has a Ka value of 9.4107. Percent ionization for a weak acid (HA) is determined by the following formula: Percentionization=[HA]initial[HA]ionized100% Part A For strong acids, ionization is nearly complete (100%) at most concentrations. However, for weak acids, the Calculate the percent ionization of HA in a 0.10M solution. percent ionization changes significantly with concentration. The Express your answer as a percent using two significant figures. more diluted the acid is, the greater percent ionization. Part B Calculate the percent ionization of HA in a 0.010M solution. Express your answer as a percent using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


