Question: Please help I will upvote! We know the rate law for a particular reaction A P is rate = 1.13x10-3 min-? [A] If the initial
![a particular reaction A P is rate = 1.13x10-3 min-? [A] If](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f84a91dd288_04966f84a914d0a8.jpg) Please help I will upvote!
Please help I will upvote!
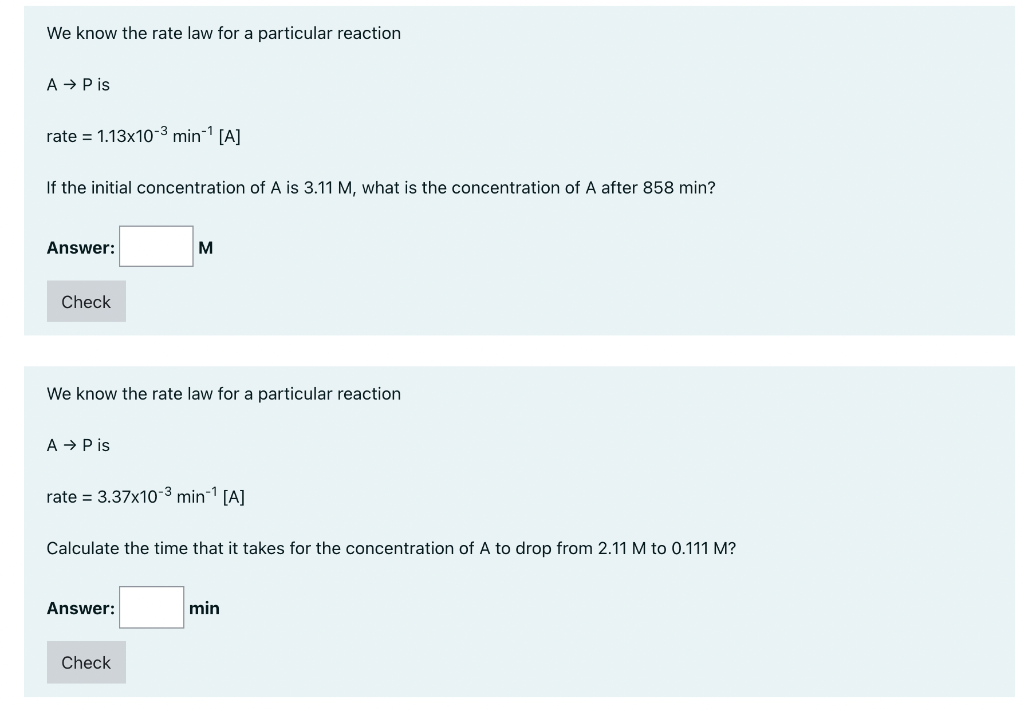
We know the rate law for a particular reaction A P is rate = 1.13x10-3 min-? [A] If the initial concentration of A is 3.11 M, what is the concentration of A after 858 min? Answer: M Check We know the rate law for a particular reaction A P is rate = 3.37x10-3 min-- [A] Calculate the time that it takes for the concentration of A to drop from 2.11 M to 0.111 M? Answer: min Check The reaction A P has the rate constant 7.86x10-3 min-!. We know that the plots of [A] versus time and 1/[A] versus time are curved lines, while a plot of In[A] versus time is a straight line. If the initial concentration of A is 2.61 M, what is the concentration of A after 89.6 minutes? Answer: M Check If the rate law for the decomposition of a drug, A, is rate = 6.03x10-3 min-? [A] calculate the time that it takes for [A] to be 38.3% decomposed. Answer: min Check
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


