Question: please help idk what i'm doing wrong The reaction below has an equilibrium constant Kp=2.2106 at 298K. 2COF2(g)CO2(g)+CF4(g) Calculate Kp for the reaction below. CO2(g)+CF4(g)2COF2(g)

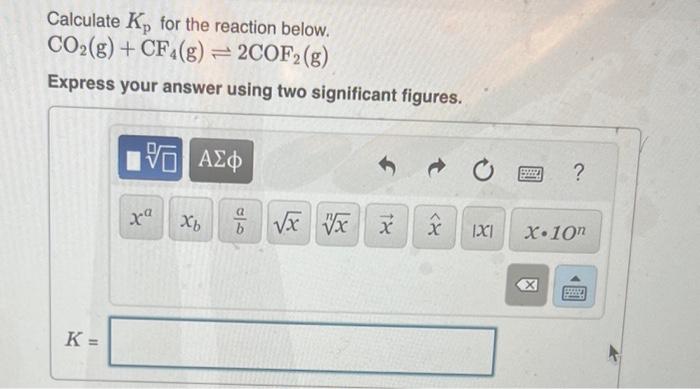
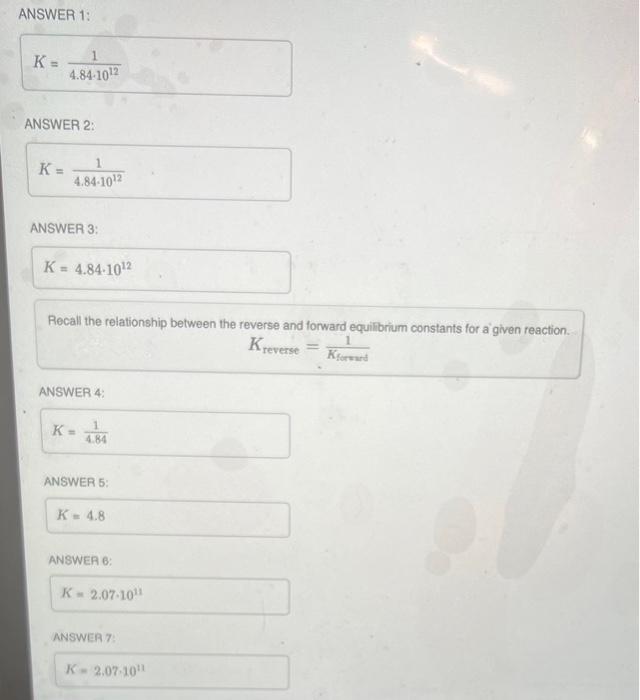
The reaction below has an equilibrium constant Kp=2.2106 at 298K. 2COF2(g)CO2(g)+CF4(g) Calculate Kp for the reaction below. CO2(g)+CF4(g)2COF2(g) Express your answer using two significant figures. ANSWER 1: K=4.8410121 ANSWER 2: K=4.8410121 ANSWER 3: Recall the relationship between the reverse and forward equint Kreverse=Kforsard1 ANSWER 4: ANSWER 5: ANSWER 6: ANSWEA 7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


