Question: The reaction below has an equilibrium constant Kp= 2.2 * 10^6 at 298 K. 2 COF2(g) CO2(g) + CF4 (g) PART A: calculate Kp for
The reaction below has an equilibrium constant Kp= 2.2 * 10^6 at 298 K. 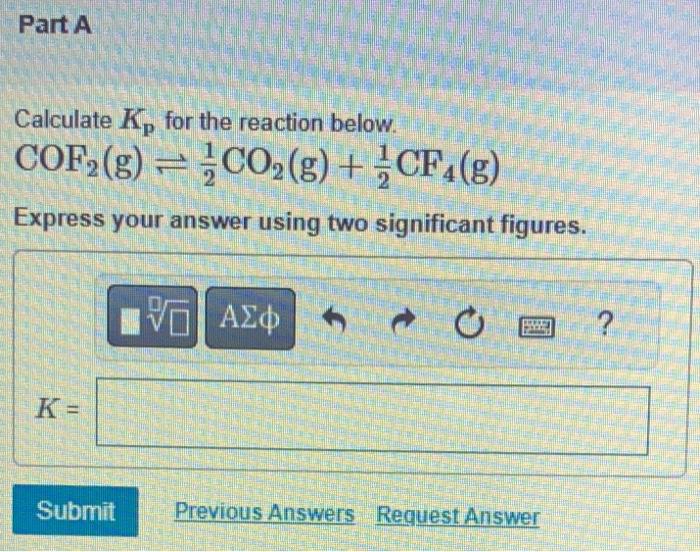
2 COF2(g) CO2(g) + CF4 (g)
PART A:
calculate Kp for the reaction below
COF2 (g) 1/2 CO2 (g) + 1/2 CF4 (g)
express answer with TWO significant figures
PART B:
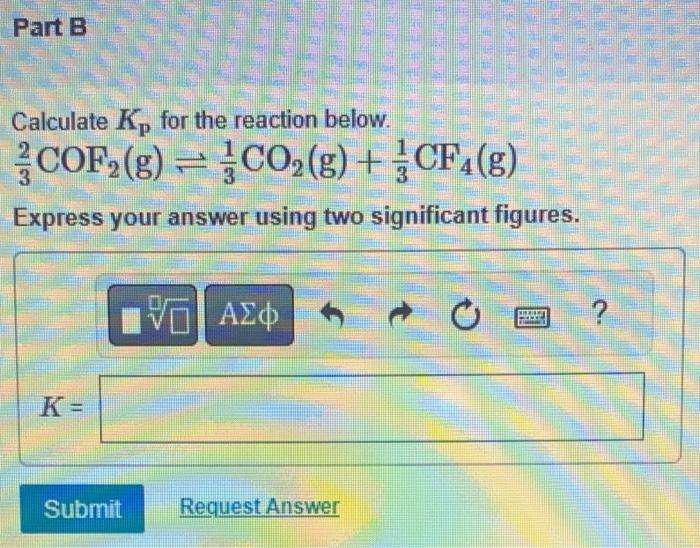
calculate Kp for the reaction below
2/3 COF2 (g) 1/3 CO2 (g) + 1/3 CF4 (g)
express answer with TWO significant figures
PART C:
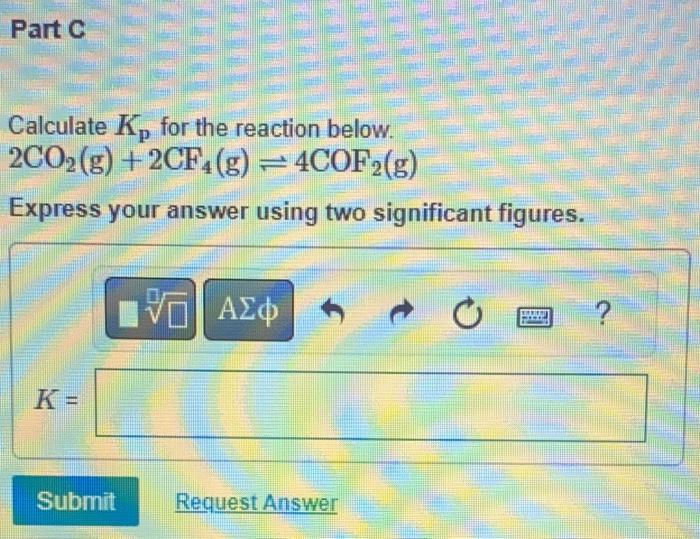
calculate Kp for the reaction below
2CO2 (g) + 2CF4 (g) 4COF2 (g)
express answer with TWO significant figures



Calculate Kp for the reaction below. COF2(g)21CO2(g)+21CF4(g) Express your answer using two significant figures. Calculate Kp for the reaction below. 32COF2(g)31CO2(g)+31CF4(g) Express your answer using two significant figures. Calculate Kp for the reaction below. 2CO2(g)+2CF4(g)4COF2(g) Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


