Question: In which of the following compounds does hydrogen bonding contribute to the intermolecular forces? Yes CH3CHO No No Yes CHF2 PH3 HF No CH5N
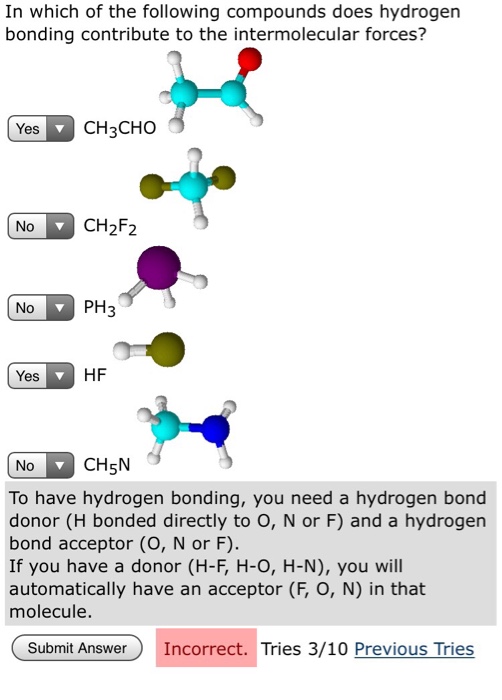
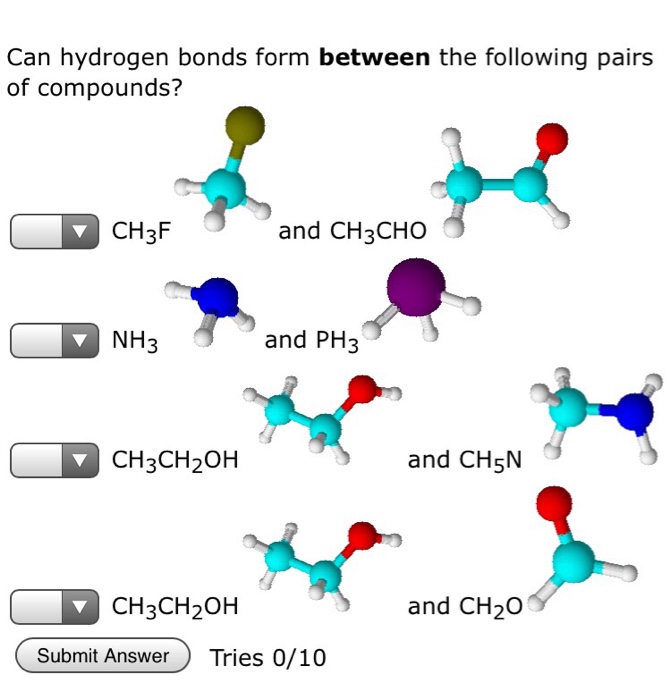
In which of the following compounds does hydrogen bonding contribute to the intermolecular forces? Yes CH3CHO No No Yes CHF2 PH3 HF No CH5N To have hydrogen bonding, you need a hydrogen bond donor (H bonded directly to O, N or F) and a hydrogen bond acceptor (O, N or F). If you have a donor (H-F, H-O, H-N), you will automatically have an acceptor (F, O, N) in that molecule. Submit Answer Incorrect. Tries 3/10 Previous Tries Can hydrogen bonds form between the following pairs of compounds? CH3F NH3 CH3CHOH CH3CHOH Submit Answer and CH3CHO and PH3 Tries 0/10 and CH5N and CHO
Step by Step Solution
3.59 Rating (156 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided below Answer 1 AcetaldehydeCH 3 CHO di fluoro ... View full answer

Get step-by-step solutions from verified subject matter experts


