Question: please help keep getting wrong In the early 1990 s, fusion involving hydrogen dissolved in palladium at room temperature, or cold fusion, was proposed as

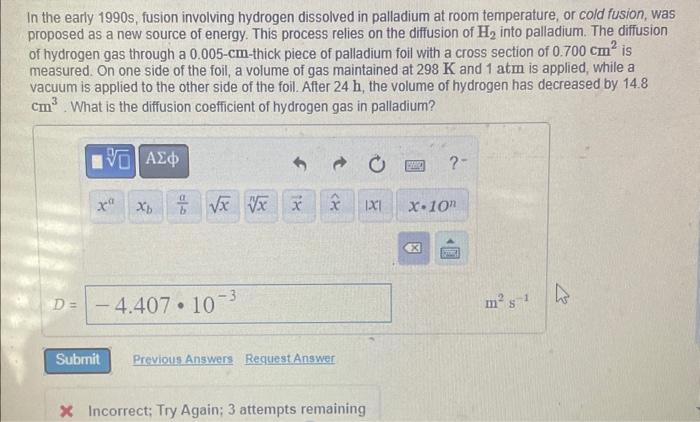
In the early 1990 s, fusion involving hydrogen dissolved in palladium at room temperature, or cold fusion, was proposed as a new source of energy. This process relies on the diffusion of H2 into palladium. The diffusion of hydrogen gas through a 0.005cm-thick piece of palladium foil with a cross section of 0.700cm2 is measured. On one side of the foil, a volume of gas maintained at 298K and 1atm is applied, while a vacuum is applied to the other side of the foil. After 24h, the volume of hydrogen has decreased by 14.8 cm3. What is the diffusion coefficient of hydrogen gas in palladium? In the early 1990 s, fusion involving hydrogen dissolved in palladium at room temperature, or cold fusion, was proposed as a new source of energy. This process relies on the diffusion of H2 into palladium. The diffusion of hydrogen gas through a 0.005cm-thick piece of palladium foil with a cross section of 0.700cm2 is measured. On one side of the foil, a volume of gas maintained at 298K and 1 atm is applied, while a vacuum is applied to the other side of the foil. After 24h, the volume of hydrogen has decreased by 14.8 cm3. What is the diffusion coefficient of hydrogen gas in palladium? C Incorrect; Try Again; 3 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


