Question: PLEASE HELP ME ANSWER ALL I WILL GIVE A THUMBS UP THANK YOU IN ADVANCE!! Use the References to access important values if needed for
PLEASE HELP ME ANSWER ALL I WILL GIVE A THUMBS UP THANK YOU IN ADVANCE!!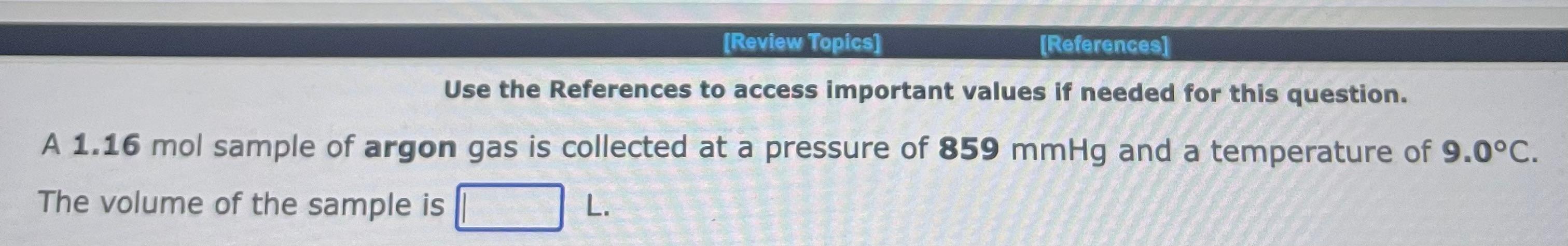
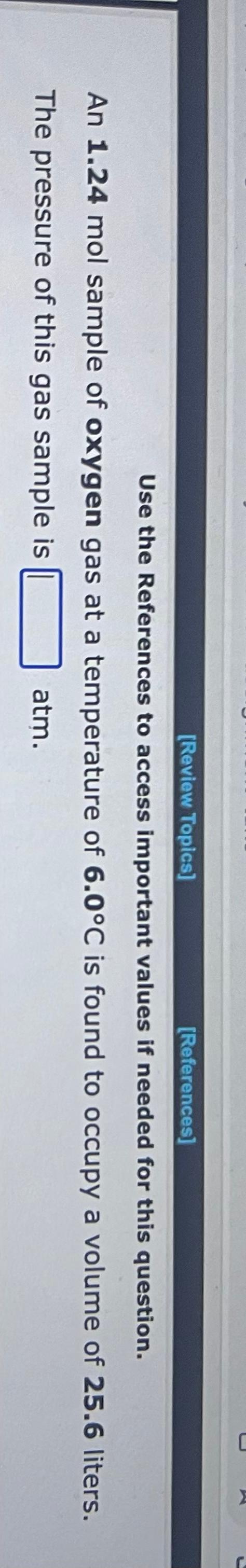
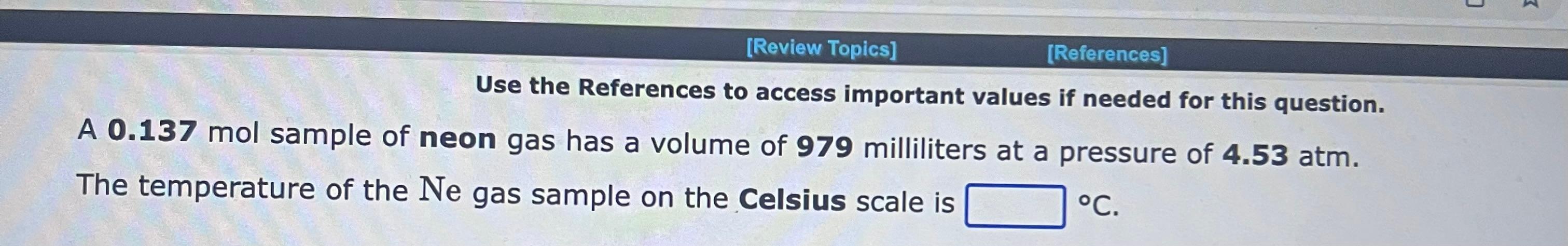
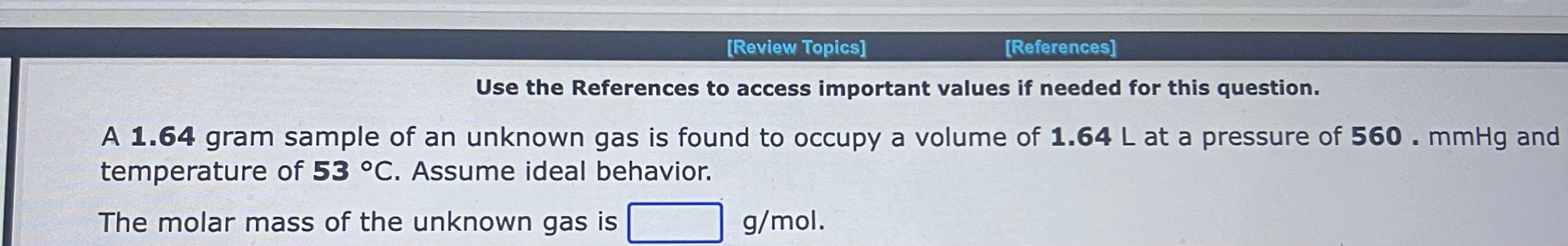
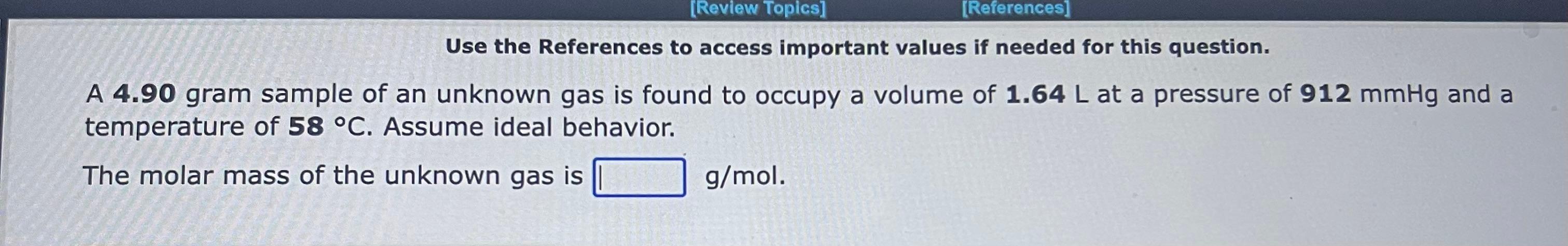
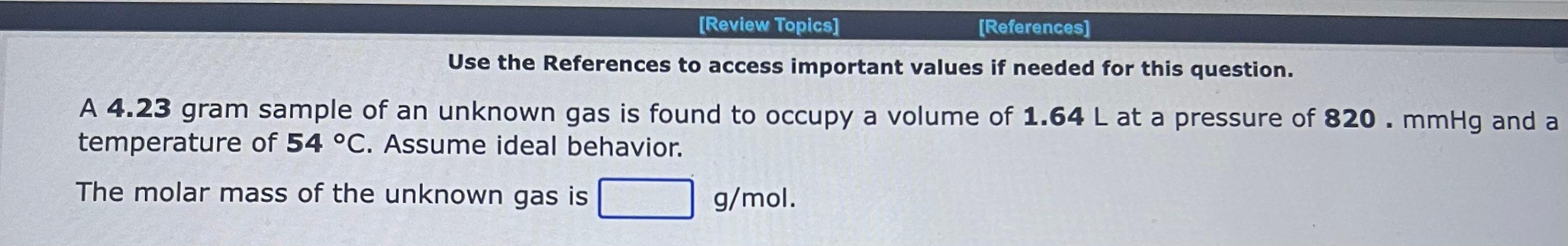
Use the References to access important values if needed for this question. A 1.16mol sample of argon gas is collected at a pressure of 859mmHg and a temperature of 9.0C. The volume of the sample is L. Use the References to access important values if needed for this question. An 1.24mol sample of oxygen gas at a temperature of 6.0C is found to occupy a volume of 25.6 liters. The pressure of this gas sample is atm. Use the References to access important values if needed for this question. A 0.137mol sample of neon gas has a volume of 979 milliliters at a pressure of 4.53atm. The temperature of the Ne gas sample on the Celsius scale is C. Use the References to access important values if needed for this question. A 1.64 gram sample of an unknown gas is found to occupy a volume of 1.64L at a pressure of 560.mmHg and temperature of 53C. Assume ideal behavior. The molar mass of the unknown gas is g/mol Use the References to access important values if needed for this question. A 4.90gram sample of an unknown gas is found to occupy a volume of 1.64L at a pressure of 912mmHg and a temperature of 58C. Assume ideal behavior. The molar mass of the unknown gas is g/mol Use the References to access important values if needed for this question. A 4.23 gram sample of an unknown gas is found to occupy a volume of 1.64L at a pressure of 820.mmHg and a temperature of 54C. Assume ideal behavior. The molar mass of the unknown gas is g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


