Question: please help me answer the 2 question i relly sont kmow what i am doing at this point . thank you very much . 1.
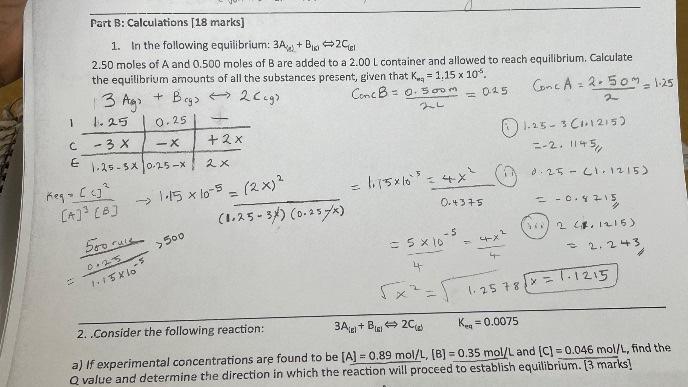
1. In the following equilibrium: 3Ae+B1812Cgi 2.50 moles of A and 0.500 moles of B are added to a 2.00L container and allowed to reach equilibrium. Calculate the equilibrium amounts of all the substances present, given that Kra=1.15105. 2. Consider the following reaction: 3A(E+B[II2C(a)Keq=0.0075 a) If experimental concentrations are found to be [A]=0.89mol/L,[B]=0.35mol/L and [C]=0.046mol/L, find the Q value and determine the direction in which the reaction will proceed to establish equilibrium. [3 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


