Question: please help me do the calculations ( the bottom part) for determination of molecular mass by freezing point lab GFi- This symbol indicates that you
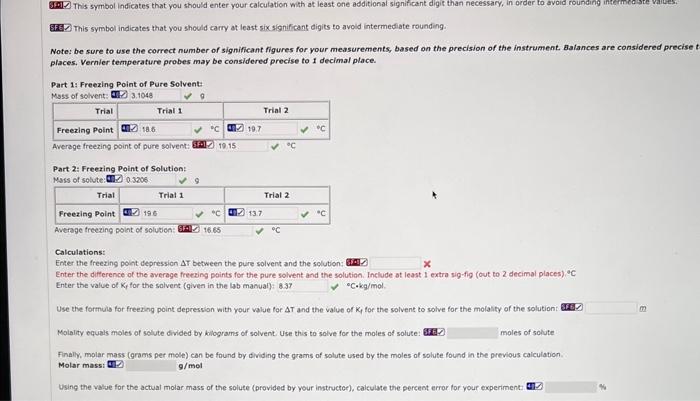
GFi- This symbol indicates that you should enter your calculation with at least one additional significant digit than necessary, in order to avoid rounding inteimediste valisest [FEC This symbol indicates that you should carry at least sixsignificant digits to avold intermediate rounding. Note: be sure to use the correct number of significant figures for your measurements, based on the precision of the instrument. Balances are considered precise t places. Vernier temperature probes may be considered precise to i decimal place. Part 14 Freexing Point of Pure Solvent: Part 2: Freezing Point of Solution: Calculations: Enter the freezing point depression AT between the pure solvent and the solution: 6.0 Enter the difference of the average freesing points for the pure solvent and the solution. Include at least I extra sig-fig (out to 2 decimal places), of Enter the value of Ky for the salvent (given in the lab manual): C-kg/mol. Use the formuls for freezing point depression with your value for T and the value of Kf for the solvent to solve for the molality of the solution: Molality equals moles of solute divided by kilograms of solvent. Use this to salve for the moles of solute: 6fifl. Finally, molar mass (arams per mole) can be found by dividing the grams of solute used by the moles of solute found in the previous caiculation. Molar massi TD g/mol Using the valse for the actual molar mass of the solute (provided by your instructer), calculate the percent error for your experimenti
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


