Question: please help me figure this out Using the data provided, find the rate constant for the reaction (with the correct units). 2NO(g)+Cl2(g)2NOCl(g) Explore the calculation
please help me figure this out
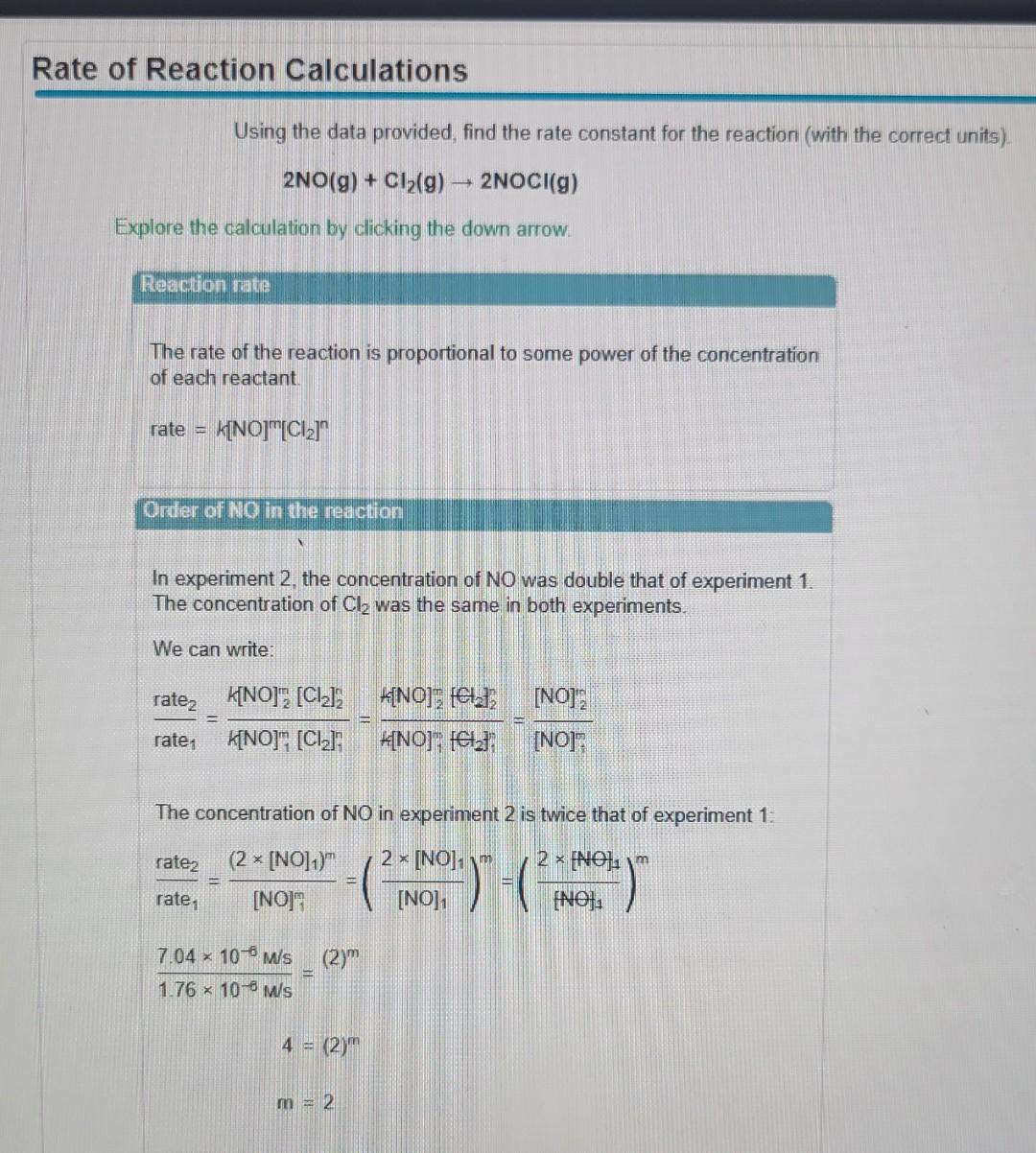
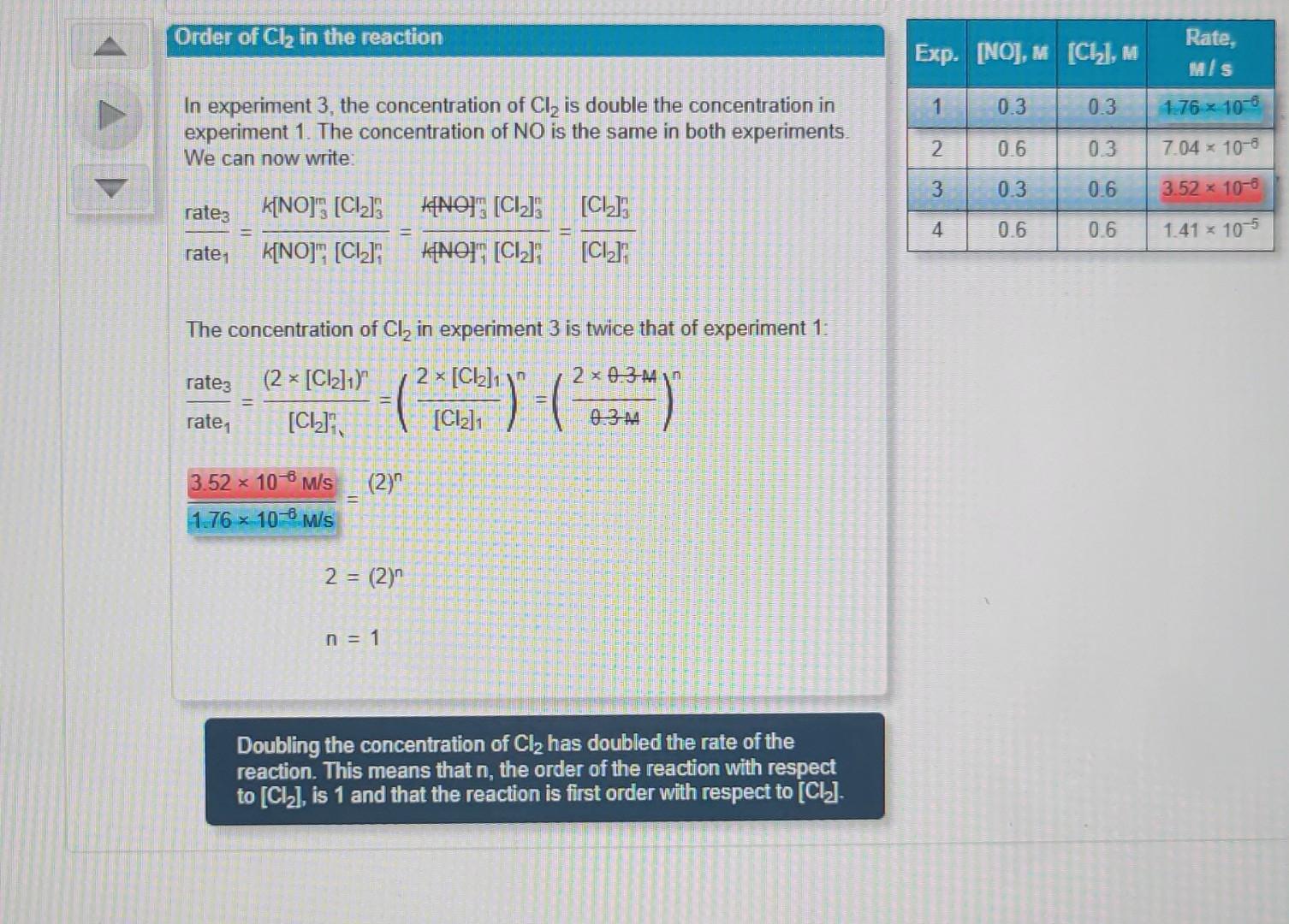
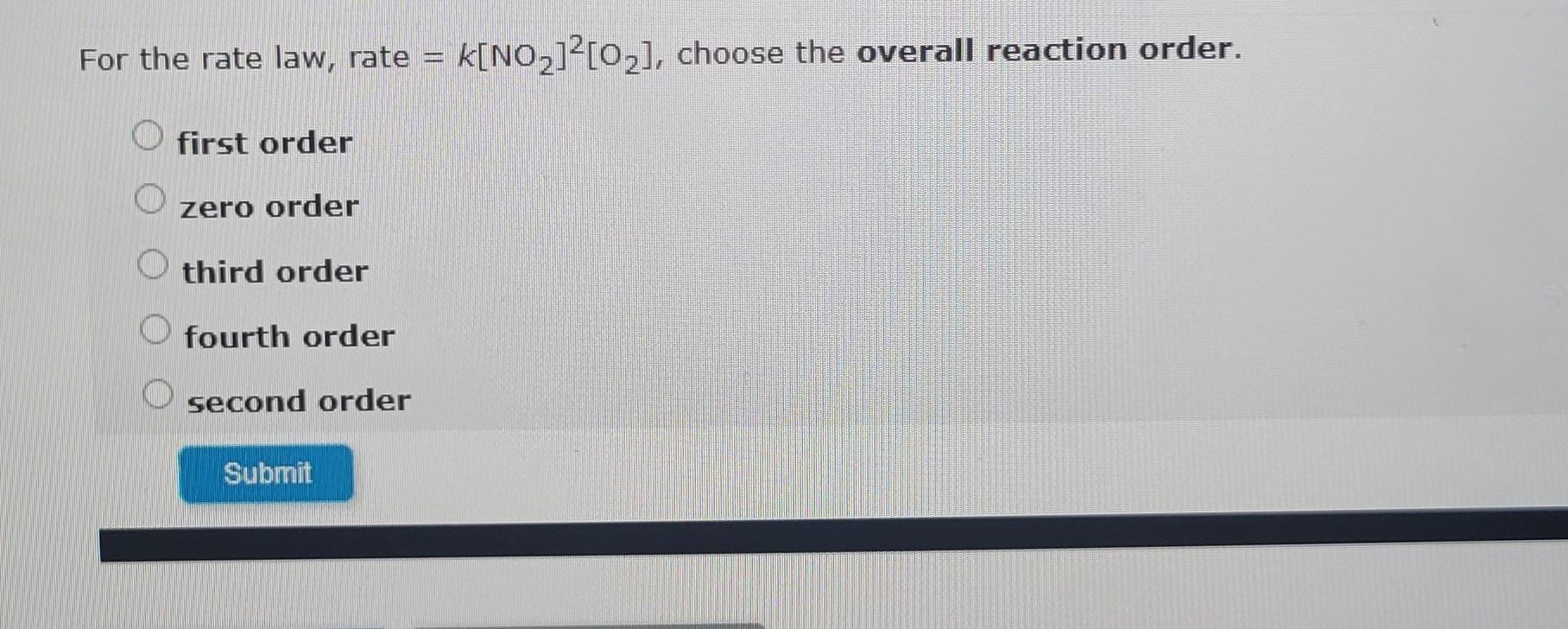
Using the data provided, find the rate constant for the reaction (with the correct units). 2NO(g)+Cl2(g)2NOCl(g) Explore the calculation by clicking the down arrow. Reaction rate The rate of the reaction is proportional to some power of the concentration of each reactant. rate=K[NO]m[Cl2]n Order of NO in the reaction In experiment 2, the concentration of NO was double that of experiment 1. The concentration of Cl2 was the same in both experiments. We can write: The concentration of NO in experiment 2 is twice that of experiment 1: rate1rate2=[NO]1m(2[NO1)m=([NO]12[NO1)m=([NO]42[NO1)m1.76106W/s7.04106W/s=(2)m4=(2)mm=2 In experiment 3 , the concentration of Cl2 is double the concentration in experiment 1. The concentration of NO is the same in both experiments. We can now write: rate1rate3=k[NO]1m[Cl2]1nk[NO]3m[Cl2]3n=k[NO1m[Cl2]1nk[NO3m[Cl2]3n=[Cl2]1n[Cl2]3n The concentration of Cl2 in experiment 3 is twice that of experiment 1: rate1rate3=[Cl2]1(2[Cl2]1)n=([Cl2]12[Cl2]1)n=(.3AA20.3AA)n1.76106M/s3.52106M/s=(2)n2=(2)nn=1 For the rate law, rate =k[NO2]2[O2], choose the overall reaction order. first order zero order third order fourth order second order
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


