Question: please help me fill out the chart! Trial 1 Trial 2 g Mass of gelatin capsule 0.114 8 0.113 % Mass of capsule and alloy
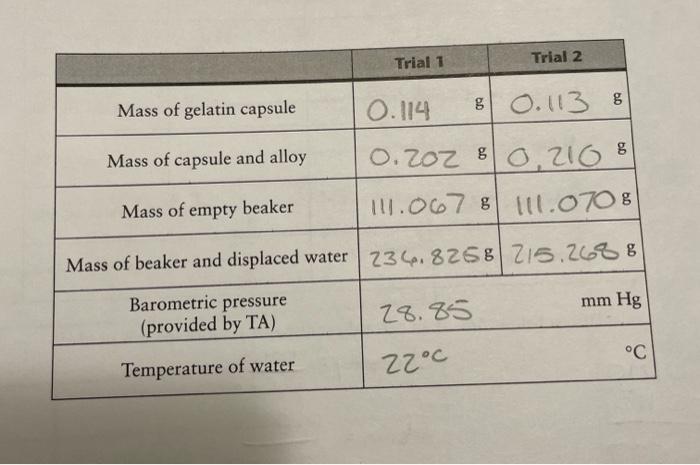
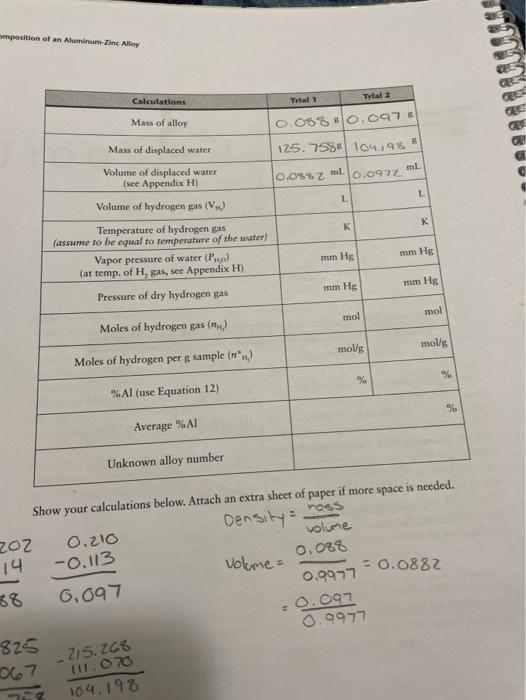
Trial 1 Trial 2 g Mass of gelatin capsule 0.114 8 0.113 % Mass of capsule and alloy 0.202 80.216 8 !!1.0678 1.070 8 Mass of empty beaker Mass of beaker and displaced water 234.8268 215.2688 mm Hg Barometric pressure (provided by TA) 28.85 C Temperature of water 22C omposition of an Aluminum-Zinc Alloy Calculations Trial 2 Mass of alloy Trial 1 0.088 0.097 125. 758 1042985 Mass of displaced water 10.0882 ml 0.0972 ml Volume of displaced water (see Appendix H) Volume of hydrogen gas (V. 1 K K mm Hg mm Hg Temperature of hydrogen gas (assume to be equal to temperature of the water) Vapor pressure of water (Pro) (at temp. of H, gas, see Appendix H) Pressure of dry hydrogen gas mm Hg mm Hg mol mol Moles of hydrogen gas () molg molg Moles of hydrogen per g sample (n.) % % % %Al (use Equation 12) % Average %AI Unknown alloy number -0.113 - 0.0882 Show your calculations below. Attach an extra sheet of paper if more space is needed. moos 202 Density 0.210 volume 14 Volume 0.088 68 6.097 0.9977 - 0.097 825 0.9977 067 104.198 - 215.268 11.070
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


