Question: please help me. I do not understand what to do A transition in the Balmer series for hydrogen has an observed wavelength of 434nm. What
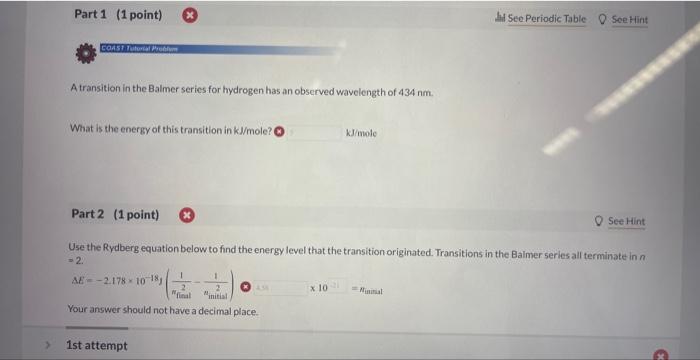
A transition in the Balmer series for hydrogen has an observed wavelength of 434nm. What is the energy of this transition in kJ/mole ? kJ/minhe Part 2 (1 point) See Hint Use the Rydberg equation below to find the energy level that the transition originated. Transitions in the Balmer series all terminate in n =2. Your answer should not have a decimal place
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


