Question: please help me i don't know how to solve it 5.- There is a standard solution of KH2PO4, which was prepared with 0.1915g of the
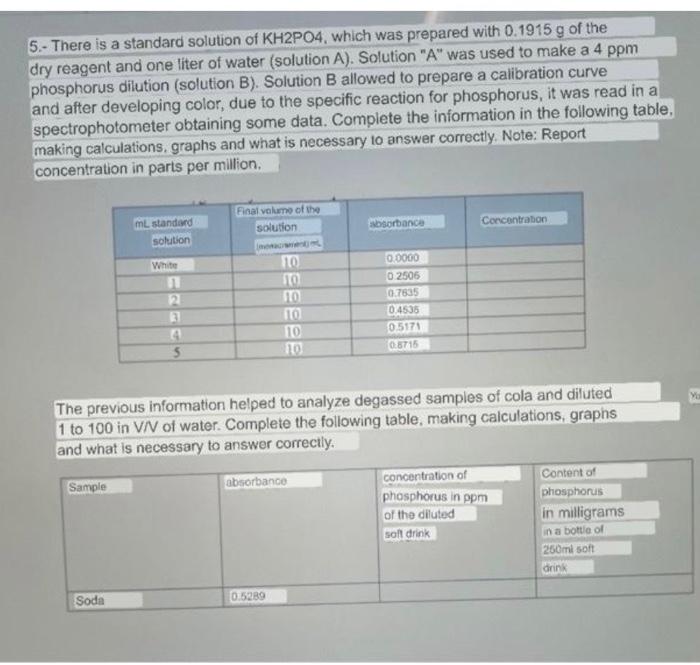
5.- There is a standard solution of KH2PO4, which was prepared with 0.1915g of the dry reagent and one liter of water (solution A). Solution "A" was used to make a 4ppm phosphorus dilution (solution B). Solution B allowed to prepare a calibration curve and after developing color, due to the specific reaction for phosphorus, it was read in a spectrophotometer obtaining some data. Complete the information in the following table, making calculations, graphs and what is necessary to answer correctly. Note: Report concentration in parts per million. The previous information helped to analyze degassed samples of cola and diluted 1 to 100 in VN of water. Complete the following table, making calculations, graphs and what is necessary to answer correctly. 5.- There is a standard solution of KH2PO4, which was prepared with 0.1915g of the dry reagent and one liter of water (solution A). Solution "A" was used to make a 4ppm phosphorus dilution (solution B). Solution B allowed to prepare a calibration curve and after developing color, due to the specific reaction for phosphorus, it was read in a spectrophotometer obtaining some data. Complete the information in the following table, making calculations, graphs and what is necessary to answer correctly. Note: Report concentration in parts per million. The previous information helped to analyze degassed samples of cola and diluted 1 to 100 in VN of water. Complete the following table, making calculations, graphs and what is necessary to answer correctly
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


