Question: Please help me in solving both parts to this problem. Thank you for your help. If a typical grain contains 0.250g of 103Pd, what is
 Please help me in solving both parts to this problem. Thank you for your help.
Please help me in solving both parts to this problem. Thank you for your help.
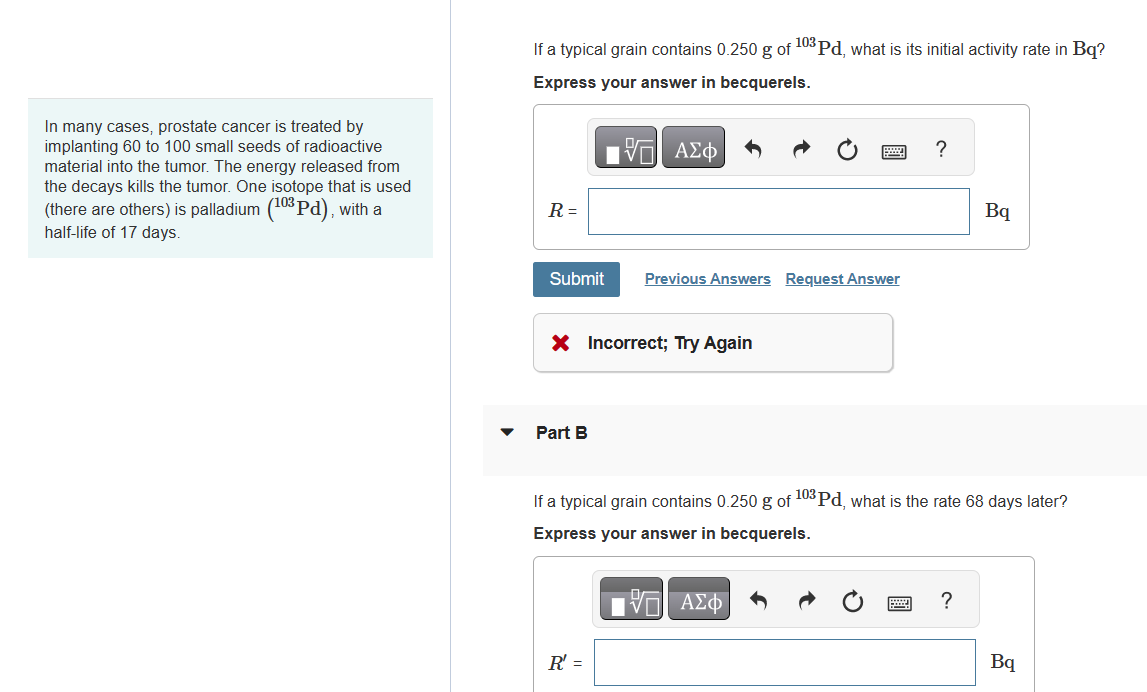
If a typical grain contains 0.250g of 103Pd, what is its initial activity rate in Bq ? Express your answer in becquerels. In many cases, prostate cancer is treated by implanting 60 to 100 small seeds of radioactive material into the tumor. The energy released from the decays kills the tumor. One isotope that is used (there are others) is palladium (103Pd), with a half-life of 17 days. X Incorrect; Try Again Part B If a typical grain contains 0.250g of 103Pd, what is the rate 68 days later? Express your answer in becquerels. If a typical grain contains 0.250g of 103Pd, what is its initial activity rate in Bq ? Express your answer in becquerels. In many cases, prostate cancer is treated by implanting 60 to 100 small seeds of radioactive material into the tumor. The energy released from the decays kills the tumor. One isotope that is used (there are others) is palladium (103Pd), with a half-life of 17 days. X Incorrect; Try Again Part B If a typical grain contains 0.250g of 103Pd, what is the rate 68 days later? Express your answer in becquerels
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


