Question: please help me = Liquids A and B when mixed form an azeotrope at 300 K and at a mole fraction XA = 0.5. It
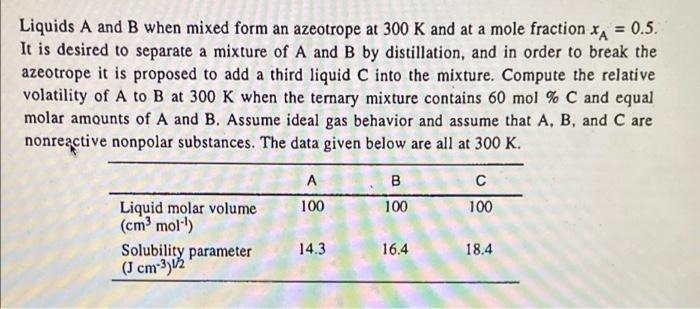
= Liquids A and B when mixed form an azeotrope at 300 K and at a mole fraction XA = 0.5. It is desired to separate a mixture of A and B by distillation, and in order to break the azeotrope it is proposed to add a third liquid C into the mixture. Compute the relative volatility of A to B at 300 K when the ternary mixture contains 60 mol % C and equal molar amounts of A and B. Assume ideal gas behavior and assume that A, B, and C are nonreactive nonpolar substances. The data given below are all at 300 K. B 100 100 100 Liquid molar volume (cm moll) Solubility parameter (J cm-3) 12 14.3 16.4 18.4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


