Separate a mixture of pyridine (left(mathrm{C}_{5} mathrm{H}_{5} mathrm{~N} ight)) and water, which form a homogeneous azeotrope, by
Question:
Separate a mixture of pyridine \(\left(\mathrm{C}_{5} \mathrm{H}_{5} \mathrm{~N}\right)\) and water, which form a homogeneous azeotrope, by using extractive distillation using bisphenol-A \(\left(\mathrm{C}_{15} \mathrm{H}_{16} \mathrm{O}_{2}\right)\) as the solvent. When finished, the system will look similar to Figure 8-14 with water as A product and pyridine as B product. The feed to column 1 is \(200.0 \mathrm{kmol} / \mathrm{h}\) of a liquid at \(80.0^{\circ} \mathrm{C}\) and \(1.0 \mathrm{~atm}\). Feed is 30.0 \(\mathrm{mol} \%\) pyridine and \(70.0 \mathrm{~mol} \%\) water. The makeup solvent is pure bisphenol-A at \(1.0 \mathrm{~atm}\) and \(100.0^{\circ} \mathrm{C}\), and the recycle solvent is also cooled to \(100.0^{\circ} \mathrm{C}\) with a heat exchanger operating at \(1.0 \mathrm{~atm}\). Produce a water product in \(\mathrm{D}_{1}\) of \(99.0 \mathrm{~mol} \%\) water or slightly higher and a pyridine product in \(\mathrm{D}_{2}\) of \(98.0 \mathrm{~mol} \%\) pyridine or slightly higher. Use nonrandom two-liquid model (NRTL) for VLE correlation. Both columns have total condensers, kettle-type reboilers, pressure constant at \(1.0 \mathrm{~atm}\), and a saturated liquid reflux.
Figure 14-8
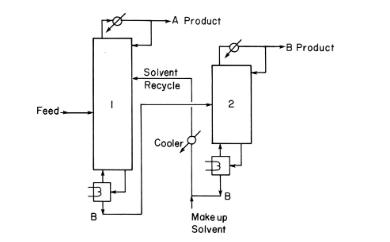
Column 1: \(\mathrm{N}=40\), makeup solvent and, when connected, recycle solvent are both on stage 3 ( \(\mathrm{N}\) and solvent feed stages will not be changed), the feed is initially on stage 30 (all in Aspen notation), and reflux ratio \(=0.1\). Set an appropriate value for the distillate flow rate to produce pure water with no water in bottoms if separation were perfect.
Column 2: \(\mathrm{N}=8\), feed is above stage 3 (all in Aspen notation), reflux ratio \(=1.0\). Set \(B_{2}\) initially to \(1000.0 \mathrm{kmol} / \mathrm{h}\). For the final design, stream \(B_{2}\) should have less than \(10^{-5}\) mole fraction of water and pyridine. Note: \(\mathrm{N}\) and the reflux ratio will not change.
The initial run will probably not give the desired purities. Find the optimum feed stage in column 1. Using this optimum feed stage and \(\mathrm{N}_{1}=\) 40 , increase the value of the solvent added by increments of \(50.0 \mathrm{kmol} / \mathrm{h}\) until the desired purities of water and pyridine are obtained.
Estimate the desired makeup rate with an external balance. Once the system is operating at the desired makeup flow rate, if necessary to obtain the desired purities, make small adjustments in the following: \(\mathrm{D}_{1}\), recycle flow rate \(\mathrm{B}_{2}, \mathrm{~L} / \mathrm{D}\) in column 1 , and makeup flow rate.
Report the following:
1. Final makeup solvent flow rate in \(\mathrm{kmol} / \mathrm{h}\)
2. Final value solvent recycle rate \(\left(B_{2}\right)\) in \(\mathrm{kmol} / \mathrm{h}\) and \(\mathrm{L} / \mathrm{D}\) in column 1 3. Final values of flow rates \(D_{1}, B_{1}\), and \(D_{2}\) in \(\mathrm{kmol} / \mathrm{h}\)
4. Mole fractions in stream \(D_{1}\)
5. Mole fractions in stream \(D_{2}\)
6. Mole fractions in stream \(B_{1}\)
7. Mole fractions in stream \(B_{2}\) (solvent recycle stream)
8. Heat load in the cooler on the solvent recycle line in cal/s
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





