Question: please help me out with question 2 and 3 please as fast as possible What is the G in kJ/mol for the reaction characterized in
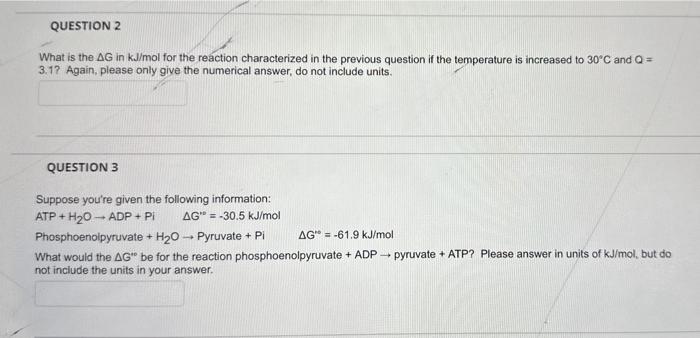
What is the G in kJ/mol for the reaction characterized in the previous question if the temperature is increased to 30C and Q= 3.1? Again, please only give the numerical answer, do not include units. QUESTION 3 Suppose you're given the following information: ATP+H2OADP+PiG=30.5kJ/mol Phosphoenolpyruvate +H2O Pyruvate +PiG0=61.9kJ/mol What would the G be for the reaction phosphoenolpyruvate + ADP pyruvate + ATP? Please answer in units of kJ/mol, but do not include the units in your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


